Search Results
Showing results 21 to 31 of 31

Forms of Carbon
Source Institutions
In this activity, educators can demonstrate how the nanoscale arrangement of atoms dramatically impacts a material’s macroscale behavior.

Got Gas?
Source Institutions
Create gas with a glass of water, some wire, conductors and a battery! You will be separating water (H2O) into oxygen and hydrogen.

Plot the Dot: A Graphical Approach to Density
Source Institutions
In this activity, learners work in groups to determine the mass and volume of four samples: glass marbles, steel washers or nuts, pieces of pine wood, and pieces of PVC pipe.

Make a Prism
Source Institutions
In this activity, learners will make their own prism and use a glass of water to separate sunlight into different colors.
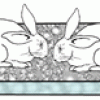
Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

Critical Angle
Source Institutions
In this optics activity, learners examine how a transparent material such as glass or water can actually reflect light better than any mirror.

Inkjet Printer
Source Institutions
In this activity, learners investigate how inkjet printers produce tiny, precise drops of ink.

Erupting Fizz
Source Institutions
This is a highly visual demonstration that illustrates both the effects of density and chemical reactions.

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.

Exploring Materials: Liquid Crystals
Source Institutions
In this activity, learners discover that the way a material behaves on the macroscale is affected by its structure on the nanoscale.

Measure the Pressure: The "Wet" Barometer
Source Institutions
In this activity, learners use simple items to construct a device for indicating air pressure changes.
