Search Results
Showing results 1 to 20 of 29

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Changing the Density of a Liquid: Adding Salt
Source Institutions
Learners see that a carrot slice sinks in fresh water and floats in saltwater.

Dissolving Different Liquids in Water
Source Institutions
In this activity, learners add different liquids to water and apply their working definition of “dissolving” to their observations.

Oily Ice
Source Institutions
In this activity, learners experiment with the density of ice, water, and oil. Learners will discover that the density of a liquid determines whether it will float above or sink below another liquid.

What's So Special about Water: Solubility and Density
Source Institutions
In this activity about water solubility and density, learners use critical thinking skills to determine why water can dissolve some things and not others.

Colors Collide or Combine
Source Institutions
Learners place multiple M&M's in a plate of water to watch what happens as the candies dissolve.

Mixing and Unmixing in the Kitchen
Source Institutions
In this chemistry investigation, learners combine common cooking substances (flour, baking powder, sugar, salt, pepper, oil, water, food coloring) to explore mixtures.

Temperature Affects Dissolving
Source Institutions
Learners design their own experiment to compare how well cocoa mix dissolves in cold and hot water. They will see that cocoa mix dissolves much better in hot water. Adult supervision recommended.

Changing the Density of an Object: Adding Material
Source Institutions
Learners see that a can of regular cola sinks while a can of diet cola floats. As a demonstration, bubble wrap is taped to the can of regular cola to make it float.
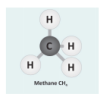
Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

Cool It!
Source Institutions
In this fun hands-on activity, learners use simple materials to investigate evaporation. How can the evaporation of water on a hot day be used to cool an object? Find out the experimental way!
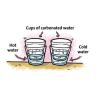
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.

Plant Power
Source Institutions
In this chemistry challenge, learners identify which plants have the enzyme "catalase" that breaks hydrogen peroxide into water and oxygen.

A Dissolving Challenge
Source Institutions
In this activity, learners add objects and substances to carbonated water to discover that added objects increase the rate at which dissolved gas comes out of solution.
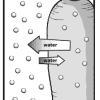
Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.
Making An Impact!
Source Institutions
In this activity (on page 14 of PDF), learners use a pan full of flour and some rocks to create a moonscape.

Scream for Ice Cream
Source Institutions
Don't scream for ice cream -- make it with milk, sugar, flavoring and some 'salt-water' ice. Discover the chemistry of ice cream by creating your own.

A Feast for Yeast
Source Institutions
In this activity on page 6 of the PDF (Get Cooking With Chemistry), learners investigate yeast. Learners prepare an experiment to observe what yeast cells like to eat.
