Search Results
Showing results 21 to 40 of 187
Liquid Layers
Source Institutions
Experiment with liquids of different densities and create liquid layers. For example, oil and water have different densities: oil floats on water because it is less dense than water.

Make a Salt Volcano (Lava Lite)
Source Institutions
This activity about density provides instructions for making a miniature "lava lite" with just salt, oil, water, and food coloring.

What's So Special about Water: Surface Tension
Source Institutions
In this three-part activity, learners play a game and conduct two simple experiments to explore water and surface tension. Learners will have fun discovering how water "sticks" together.

Frozen Sculptures
Source Institutions
In this activity, learners use objects they find on a nature and water to make creative frozen sculptures.

Water Drop Exploration
Source Institutions
In this activity learners will explore how water moves on waxed paper. Learners will use science process skills such as using tools and making observations as they explore surface tension.

Gravity-Defying Water
Source Institutions
In this activity, learners explore gravity and air pressure as they experiment with holding a glass full of water upside down, without spilling it, using a simple piece of cardstock.
Floating Paperclip and Other Surface Tension Experiments
Source Institutions
In this activity, learners experiment with surface tension using everyday household items such as strawberry baskets, paperclips, liquid dish soap, and pepper.

Convection Current
Source Institutions
In this activity, learners make their own heat waves in an aquarium.
Instant Ice
Source Institutions
In this activity, learners observe a quick phase change as water rapidly goes from a liquid state to a solid state.

Oily Ice
Source Institutions
In this activity, learners experiment with the density of ice, water, and oil. Learners will discover that the density of a liquid determines whether it will float above or sink below another liquid.

How Much Water is in that Cloud?
Source Institutions
In this activity, learners working in pairs saturate a cotton ball using water drops from an eyedropper to demonstrate the high water capacity of clouds.

What's So Special about Water: Solubility and Density
Source Institutions
In this activity about water solubility and density, learners use critical thinking skills to determine why water can dissolve some things and not others.

The Rain Man
Source Institutions
In this activity, learners observe the hydrologic cycle in action as water evaporates and condenses to form rain right before their eyes.

Wiggly Water
Source Institutions
This is a simple and fun activity for learners to explore water and colors.
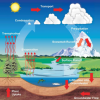
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

Twist and Spout
Source Institutions
In this activity, learners make their own "tornado" using two soda bottles and water.
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.
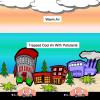
Turning the Air Upside Down: Convection Current Model
Learners see convection currents in action in this highly visual demonstration. Sealed bags of colored hot or cold water are immersed in tanks of water.
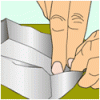
Aluminum Boats
Source Institutions
Test the buoyancy of an aluminum foil boat and an aluminum foil ball. Why does the same material in different shapes sink or float?
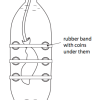
Submersibles and Marshmallows
Source Institutions
In this activity, learners discover the difficulty of ocean exploration by human beings as they investigate water pressure.
