Search Results
Showing results 1 to 20 of 114

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Go With the Flow
Source Institutions
In this activity, learners will observe laminar and turbulent flow of water using only a plastic bottle, liquid hand soap, food coloring and water.

Water, Water Everywhere
Source Institutions
In this activity, learners estimate how much water they think can be found in various locations on the Earth in all its states (solid, liquid, and gas) to discover the different water ratios in the Ea

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.

Soapy Boat
Source Institutions
Learners discover that soap can be used to power a boat. Learners make a simple, flat boat model, put it in water, and then add a drop of detergent at the back of the boat.

Levity Through Tension: Fun with Water's Surface Tension
Source Institutions
This experiment describes how to create a "dribble bottle" which only leaks water when the cap is unscrewed. The full water bottle has a small hole made with a push pin.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.
Drip Drop
Source Institutions
In this water activity, learners explore how water drops behave on different surfaces.

From Here to There
Source Institutions
In this water activity, learners discover ways to move water across the water table.

Drop Shape
Source Institutions
In this activity, learners get a closer look at the shape of a drop of water and a drop of oil. Learners first drip water onto wax paper and examine the shape of separate drops from a side view.

Penny Drop
Source Institutions
In this quick activity about the properties of water (page 1 of PDF under SciGirls Activity: Malformed Frogs), learners will use an eyedropper to slowly place one drop of water at a time onto a penny,

Changing the Density of a Liquid: Heating and Cooling
Source Institutions
Learners investigate how the temperature of water affects its density.

Sunny Day Painting
Source Institutions
In this activity, learners explore properties of water and watch evaporation happen by "painting" with water in the sun.

Make a Wire Critter That Can Walk on Water
Source Institutions
In this activity, learners make water-walking critters using thin wire, and then test how many paper clips these critters can carry without sinking.
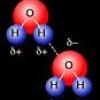
Cohesion Coin
Source Institutions
In this activity about the property of water (page 6 of the PDF), learners use a coin to demonstrate cohesion between water molecules, exploring the molecular forces that allow water molecules to "
What's So Special about Water: Absorption
Source Institutions
In this activity about water's cohesive and adhesive properties and why water molecules are attracted to each other, learners test if objects repel or absorb water.

Frosty Glasses
Source Institutions
In this activity, learners explore why frost forms. They create their own frost using a solution of ice water and salt in a glass.

Dissolving Different Liquids in Water
Source Institutions
In this activity, learners add different liquids to water and apply their working definition of “dissolving” to their observations.
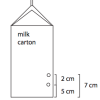
Pressing Pressure
Source Institutions
In this activity, learners compare water pressure at different depths. Learners discover that water pressure increases with depth.
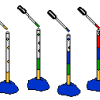
Liquid Layers
Source Institutions
Experiment with liquids of different densities and create liquid layers. For example, oil and water have different densities: oil floats on water because it is less dense than water.
