Search Results
Showing results 281 to 300 of 320

Copper Caper
Source Institutions
In this activity, learners conduct an oxidation experiment that turns old pennies bright and shiny. Learners soak 20 dull, dirty pennies in a bowl of salt and vinegar for five minutes.
River Catcher
Source Institutions
In this activity (located at the top of the page), learners make an easy river strainer and see what they can catch.
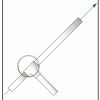
Launch Altitude Tracker
Source Institutions
In this activity, learners construct hand-held altitude trackers. The device is a sighting tube with a marked water level that permits measurement of the inclination of the tube.

Plankton Feeding
Source Institutions
This activity provides a hands-on experience with a scale model, a relatively high viscosity fluid, and feeding behaviors.

Do Cities Affect the Weather?
Source Institutions
In this activity, learners explore clouds and how they form.

Exploring Earth: Investigating Clouds
Source Institutions
“Exploring Earth: Investigating Clouds” is a hands-on activity in which visitors create a cloud in a bottle and explore it with laser light.

Iridescent Art
Source Institutions
This is a quick activity (on page 2 of the PDF under Butterfly Wings Activity) that illustrates how nanoscale structures, so small they're practically invisible, can produce visible/colorful effects.

Rubber Blubber Gloves
Source Institutions
In this experiment, learners work in pairs to create two gloves -- one that contains a layer of shortening (blubber) inside, and one that doesn't.

Mystery Matter
Source Institutions
This interactive demonstration reintroduces learners to three states of matter (solid, liquid, gas), and introduces them to a fourth state of matter, plasma.

Hot Stuff!: Testing Ice
In this demonstration, learners compare and contrast regular water ice to dry ice (frozen carbon dioxide). Both samples are placed in a solution of acid-base indicator.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Iodine Investigators!
Source Institutions
In this activity on page 7 of the PDF (Chemistry—It’s Elemental), learners use iodine to identify foods that contain starch.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.

Resistance is Useful
Source Institutions
Learners write or draw with white crayon on white paper. They look and feel to detect their marks on the paper. Then, learners paint over their paper with watercolor paint.

Sea Level: On The Rise
Source Institutions
Learners will understand the relationship between climate change and sea-level rise.

Cabbage Juice Indicator: Test the pH of household products
Source Institutions
Learners make their own acid-base indicator from red cabbage. They use this indicator to test substances around the house.
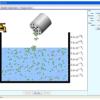
Salts & Solubility
Source Institutions
In this online interactive simulation, learners will add different salts to water and then watch the salts dissolve and achieve a dynamic equilibrium with solid precipitate.

ZOOM Glue
Source Institutions
In this activity, learners mix milk, vinegar, baking soda, and water to create sticky glue. Use this activity to explain how engineers develop and evaluate new materials and products.

Shipping for Survival
Source Institutions
In this activity, learners explore how packaging engineers develop customized shipping and packaging containers to meet the needs of many different industries.

Gelatin Used for Drug Delivery
Source Institutions
In this activity, learners discover how gelatin can be used as a medium for drug delivery. Learners create colored gelatin and then cut out pieces of the gelatin to simulate medicine (pills).
