Search Results
Showing results 281 to 300 of 301

Keep it Cool
Source Institutions
In this activity, learners explore how engineers have met the challenge of keeping foods, liquids, and other items cool.

Surface Area
Source Institutions
In this demonstration, learners discover that nanoparticles behave differently, in part because they have a high surface area to volume ratio.
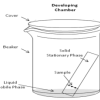
Separating with Chromatography
Source Institutions
In this experiment, learners separate different types of molecules in marker inks (using a technique called "thin layer chromatography").

Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.

Disappearing Statues
Source Institutions
In this activity (on page 8), learners model how marble statues and buildings are affected by acid rain.

Monitoring Amphibians
Source Institutions
In this field study, learners discover how to collect data in the field and how their efforts can help certain animals, specifically, amphibians.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

Wild Sourdough
Source Institutions
In this activity, learners explore chemistry and the microbial world by making their own sourdough starter and bread at home using only flour and water.
Beam Me Up!
Source Institutions
This is a quick activity (on page 2 of the PDF under Stained Glass Activity) about the "Tyndall effect," the scattering of visible light when it hits very small dispersed particles.

Fizzy Nano Challenge
Source Institutions
This lesson focuses on how materials behave differently as their surface area increases.

Chromatography Can Separate!
Source Institutions
In this chemistry activity, learners use thin layer chromatography to determine the molecular composition of different markers.

Environmental Chemistry
Source Institutions
In this activity with several mini experiments, learners explore the chemistry that helps scientists learn about the environment and how they can help save it.

Oil Spill Solutions
Source Institutions
In this activity, learners explore how environmental engineers might approach solving the problem of an oil spill.

Cook Up a Comet
Source Institutions
In this activity (on page 5 of PDF), learners use dry ice and household materials to make scientifically accurate models of comets.

Photosynthetic Pictures Are Worth More Than a Thousand Words
Source Institutions
This activity provides an opportunity for learners to observe and examine how carbon dioxide, water, and light produce glucose/starch through a process called photosynthesis.

Demonstrating An Epidemic
Source Institutions
This experiment allows learners to experience a small scale "epidemic," demonstrating the ease with which disease organisms are spread, and enables learners to determine the originator of the "epidemi

Carrying Charges: Testing for Conductivity
Source Institutions
Learners are challenged to create solutions that conduct electricity and make a buzzer buzz (or an LED light up).

Biochemistry Happens Inside of You!
Source Institutions
In this four-part activity, learners explore how the body works and the chemistry that happens inside living things.

Super Soil
Source Institutions
In this outdoor activity, learners make their own organic-rich soil. Depending on where this activity is done, learners will probably discover that their local soil is low in organic matter.
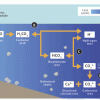
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.
