Search Results
Showing results 41 to 60 of 301

What's So Special about Water: Solubility and Density
Source Institutions
In this activity about water solubility and density, learners use critical thinking skills to determine why water can dissolve some things and not others.

What's in the Water
Source Institutions
"What's in the Water" lets participants use tools to solve the mystery- what chemicals and compounds are in a sample of water?

Wiggly Water
Source Institutions
This is a simple and fun activity for learners to explore water and colors.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.

Diet Light
Source Institutions
In this quick activity, learners observe how the added sugar in a can of soda affects its density and thus, its ability to float in water.

Release the Grease!
Source Institutions
In this simple activity (on page 7 of the PDF), learners use water and liquid dish detergent to see which one removes lipstick better from an index card.
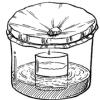
Rain Machine (Solar Still)
Source Institutions
In this activity, learners work in groups to build simple solar stills filled with salt water. After the stills are complete, learners observe what happens when they place the stills in the sun.
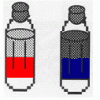
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Dye Detective
Source Institutions
Learners use filter paper and water to analyze six different markers. They mark the paper with ink, and dip the paper in water. The water travels up the paper and dissolved ink travels with it.

Color-Changing Carnations
Source Institutions
Learners place cut flowers in colored water and observe how the flowers change. The flowers absorb the water through the stem and leaves.

Dye Detective
Source Institutions
Learners analyze mixtures of dyes using filter paper chromatography. They place spots of the different dyes at the bottom of a piece of filter paper, and hang the paper to touch the surface of water.

Crystal Creations: Grow Spikes of Crystals in the Sun
Source Institutions
This activity shows you how to make amazing crystal spikes using Epsom salt and the sun.
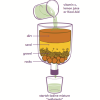
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Water Body Salinities II
Source Institutions
In this activity, learners discuss the different salinities of oceans, rivers and estuaries.

What is in the Water?
Source Institutions
In this activity, learners use open inquiry to learn about the process of science as well as gain experience regarding the Law of Conservation of Mass, dissolution, and density.

Ocean in a Bottle
Source Institutions
In this simulation activity, learners observe what can happen when ocean waves churn up water and oil from an oil spill.

Causes and Effects of Melting Ice
Source Institutions
In this activity, learners explore the concept of density-driven currents (thermohaline circulation) and how these currents are affected by climate change.

Forces at the Nanoscale: Nano Properties of Everyday Plants
Source Institutions
This is an activity (located on page 3 of PDF under Nasturtium Leaves Activity) about surface tension.

Colors Collide or Combine
Source Institutions
Learners place multiple M&M's in a plate of water to watch what happens as the candies dissolve.
