Search Results
Showing results 361 to 380 of 414

Bone Basics
Source Institutions
This is an activity (on page 2 of the PDF under Bone Regrowth Activity) about the two main components of bone - collagen and minerals (like calcium) - and how they each contribute to its flexibility a

Jell-O Model of Microfluidics
Source Institutions
This activity uses Jell-O(R) to introduce learners to microfluidics, the flow of fluids through microscopic channels.

Shipping for Survival
Source Institutions
In this activity, learners explore how packaging engineers develop customized shipping and packaging containers to meet the needs of many different industries.

Why Circulate?
Source Institutions
In this activity related to the human circulatory system (on page 10 of the PDF), learners observe the dispersion of a drop of food coloring in water, draw conclusions about the movement of dissolved

Dunking the Planets
Source Institutions
In this demonstration, learners compare the relative sizes and masses of scale models of the planets as represented by fruits and other foods.
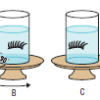
Visualizing How the Vestibular System Works
Source Institutions
In this activity (page 59 of the PDF), learners spin and observe false eyelashes in jars of water (prepared at least 1 day ahead of time) to investigate the effects of different types of motion on the

Color Me Blue
Source Institutions
In this activity, learners add dilute bleach solution to water that has been dyed with yellow, blue, and green food color.

Underwater Hide and Seek
Source Institutions
In this activity, learners experience firsthand how marine animals' adaptive coloration camouflages them from prey.

Spaghetti Strength
Source Institutions
In this activity on page 7 of the PDF, learners explore how engineers characterize building materials.

Sugar Crystal Challenge
Source Institutions
This lesson focuses on surface area and how the shape of sugar crystals may differ as they are grown from sugars of different coarseness.
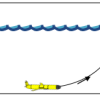
Exploring the Ocean with Robots
Source Institutions
In this activity, learners are introduced to robotic submarines called gliders. Learners make “gliders” from plastic syringes and compare these to Cartesian bottles and plastic bubbles.

Exploring A Hydrogel
Source Institutions
In this activity on page 10 of the PDF, learners develop an experiment to answer the following question: "How much water can the hydrogel in a baby diaper hold?" Use this activity to explore polymers,

Marine Ecosystems
Source Institutions
In the wild, small crustaceans known as brine shrimp live in marine habitats such as saltwater lakes.

Soggy Science, Shaken Beans
Source Institutions
Learners explore soybeans, soak them in water to remove their coat, and then split them open to look inside. They also make a musical shaker out of paper cups, a cardboard tube, and soybeans.

Candy Chemistry
Source Institutions
In this experiment, learners test multiple food items to see if they are an acid or base using an indicator solution created with red cabbage.

Potion Commotion
Source Institutions
In this hands-on science experiment, students combine their understanding of the different states of matter and the characteristics of various chemical reactions.

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.

Currently Working
Source Institutions
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution.

Making Sense of Sensors
Source Institutions
In this activity, learners explore sensors and focus specifically on how to measure humidity using a sensor.

Frozen Fruit
Source Institutions
In this "Sid the Science Kid" activity from Episode 108: My Ice Pops, learners observe reversible change while thinking about ways to make ice melt.
