Search Results
Showing results 821 to 840 of 911

Flubber: Make a polymer!
Source Institutions
This activity (on page 2 of the PDF) features a recipe to create the stretchy polymer Flubber from Borax detergent, white glue, and water.

Sand Paper Rankings
Source Institutions
In this activity (2nd activity on the page), learners explore the sensitivity of their sense of touch.

Outbreak!: Investigating Epidemics
Source Institutions
A group of learners simulates the spread of disease by trading slips of paper, one set of which has been treated with a baking soda solution.

Making Circuits
Source Institutions
In this activity, learners explore electricity and conductivity to find that many things conduct electricity including copper, pencil lead, fruit, play-doh, and even people!

Keeps on Pumpin': Your Heart
Source Institutions
In this activity, learners explore the great pump in their chests--the human heart!

Plaster Casts
Source Institutions
In this activity, learners combine two substances (plaster of Paris and water) to make a cast of an object's imprint in clay.

Shaking It!
Source Institutions
In this experiment, learners design and build a model room in a shoebox and furnish it with tiny furniture.

Change in Temperature: Exothermic Reaction
Source Institutions
Learners add calcium chloride to a baking soda solution and observe an increase in temperature along with the production of a gas and a white precipitate. These are all signs of a chemical reaction.

Gelatin Prism
Source Institutions
In this activity, learners make prisms from gelatin. Learners then shine light through the prisms and discover what happens. This activity introduces learners to the idea of refraction.

Slime
Source Institutions
Learners make slime from white school glue and Borax detergent. The long chain molecules of the white glue become cross-linked by the Borax into a big network.
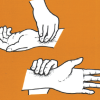
Cardiac Hill
Source Institutions
In this outdoor activity linking human health to the environment, learners use their pulse rates as a measure of the effort expended in walking on different slopes.
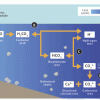
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.

Floating Dry Erase Creations
Source Institutions
In this activity, learners will create a drawing with dry erase markers and watch it come to life. Learners will explore chemistry, art and storytelling through this activity.
Four Corners
Source Institutions
In this design challenge activity, learners build a machine out of cardboard that runs smoothly and dependably. Learners must be precise to make sure their component works properly.

Fruity Electricity
Source Institutions
In this activity, Frankenstein's lab is running out of electricity! Learners use fruit to help Igor find a temporary source of energy to turn on a light.

Make a Comeback Can
Source Institutions
Learners build a can that automatically returns after being rolled away. The can has a rubber band inside that stores energy as the can rolls one direction.

Create Gas
Source Institutions
Learners mix vinegar and baking soda together in a bottle to create a chemical reaction. The reaction produces a gas, carbon dioxide, which inflates a balloon attached to the mouth of the bottle.

The Unpoppable Balloon
Source Institutions
In this activity, learners explore polymer structure and their ability to reform around objects by attempting to stab a wooden skewer through a balloon without popping it.

The Carbon Cycle and its Role in Climate Change: Activity 3
Source Institutions
In this activity, learners explore the human influences on the carbon cycle and examine how fossil fuels release carbon.

How Big is Small
Source Institutions
In this classic hands-on activity, learners estimate the length of a molecule by floating a fatty acid (oleic acid) on water.
