Search Results
Showing results 1 to 20 of 31

Cleaning with Dirt
Source Institutions
Learners build a filter from old soda bottles and dirt. They create polluted water, and pour it through their filter to clean it.
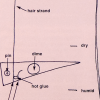
Better Hair Through Chemistry
Source Institutions
In this activity, learners hook up a hair to a lever system and create a hair hygrometer to measure changes in humidity.

Separation Anxiety
Source Institutions
In this activity, learners discover the primary physical properties used to separate pure substances from mixtures.

Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.

DNA Extraction: Look at your genes!
Source Institutions
Extract your DNA from your very own cells! First, learners swish salt water in their mouth to collect cheek cells and spit the water into a glass.

Chemistry is Colorful
Source Institutions
In this activity, learners explore materials through paper chromatography.

Finding Red
Source Institutions
In this chemistry challenge, learners systematically investigate which combination of four solutions produces a deep red color.

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.

Be A Pasta Food Scientist
Source Institutions
In this activity, learners of all ages can become food scientists by experimenting with flour and water to make basic pasta.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Natural Indicators
Source Institutions
Learners combine different plant solutions -- made from fruits, vegetables, and flowers -- with equal amounts of vinegar (acid), water (neutral), and ammonia (base).

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

Flubber
Source Institutions
Learners experiment with a piece of Silly Putty® by stretching, bouncing, and snapping it. They then create flubber, a similar substance, by mixing diluted glue and a solution of sodium borate.

Watercolor
Source Institutions
In this activity, learners will use chemistry to create a night sky watercolor painting. They will experiment to learn the effects of mixing crayon, salt, and lemon juice with water color paints.

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.

Oil Spill Solutions
Source Institutions
In this activity, learners explore how environmental engineers might approach solving the problem of an oil spill.
