Search Results
Showing results 41 to 60 of 65

Burning Issues
Source Institutions
Learners use a candle to investigate the products of combustion. When a glass rod is held over a lit candle, the candle flame deposits carbon on the rod.

Rock Bottoms
Source Institutions
Learners add acid rain (nitric acid) to two cups that represent lakes. One cup contains limestone gravel and the other contains granite gravel.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.
Foam Peanuts
Source Institutions
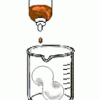
Learners compare the properties and solubilities of Styrofoam (TM), ecofoam packing peanuts, and popcorn. First, the solubility of each substance is tested in water.
Electrolysis
Source Institutions
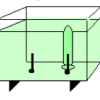
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.
Acid Rain Eats Stone!
Source Institutions
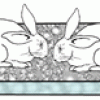
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.
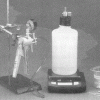
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Currently Working
Source Institutions
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution.

Slide Rules
Source Institutions
Learners make their own simple slide rules out of paper and learn how they work.
Tricky Tangrams
Source Institutions
In this activity (on pages 49-54 of PDF), learners play with tangrams, a set of triangles, squares and a parallelogram that can combine into a larger square as well as all sorts of other shapes.

See the Light
Source Institutions
Learners mix a solution of luminol with hydrogen peroxide to produce a reaction that gives off blue light.

Bounce vs. Thud Balls
Source Institutions
Learners compare the properties of two balls that appear identical. One ball bounces, while the other ball "thuds." The “bounce” ball is made of the polymer polybutadiene (-C4H4-).

Fireworks!
Source Institutions
In this chemistry lab activity, learners model the colors of fireworks by burning metallic solutions in a flame and observing the different colors produced.

Jelly Beads
Source Institutions
Learners add drops of alginate solution to a solution of calcium chloride. The alginate does not mix with the calcium chloride, but forms soft gel beads.

Dusted!
Source Institutions
Learners press their fingertip onto a clean Plexiglas sheet. The fingerprints are then revealed as learners dust over the print with fingerprint powder.

Lava Lamps
Source Institutions
Learners observe working lava lamps to understand how they work (included in PDF link).
First Impressions
Source Institutions
Learners experiment with a commercial photo-sensitive paper (Sunprint® or NaturePrint® paper). They place opaque and clear objects on the paper and expose it to bright light, observing the results.

Magical Möbius
Source Institutions
In this tabletop activity (on pages 32-40), learners make Möbius strips -- 3D surfaces with only one side.

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.
