Search Results
Showing results 1 to 20 of 21

Rainbow Paper
Source Institutions
In this activity, learners will use clear nail polish and the power of chemistry to create paper with a rainbow sheen.

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Make Your Own Sculpture Dough
Source Institutions
In this activity on page 7 of the PDF, learners follow a recipe to make a dough similar to the clay artists use to make sculptures.
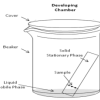
Separating with Chromatography
Source Institutions
In this experiment, learners separate different types of molecules in marker inks (using a technique called "thin layer chromatography").

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.
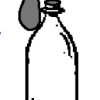
Gas Production: Blow up a balloon!
Source Institutions
In this classic reaction, learners baking soda and vinegar in a soda bottle to produce carbon dioxide (CO2) gas. This gas inflates a balloon.

Homemade Bath Fizzies
Source Institutions
In this activity, learners make their own bath bomb fizzies and experience what happens when they mix a base and an acid.

Fizzy Foam Fun
Source Institutions
In this activity, learners create a colorful foam fountain by adding yeast to a chemical reaction. This activity introduces chemical reactions to young learners and teaches the concept of catalysts.

Chemistry is Colorful
Source Institutions
In this activity, learners explore materials through paper chromatography.

DIY Elephant Toothpaste
Source Institutions
In this activity, learners will experiment with catalysts to create an at-home version of elephant toothpaste.

Disappearing Statues
Source Institutions
In this activity (on page 8), learners model how marble statues and buildings are affected by acid rain.

Changing Colors
Source Institutions
Learners experiment with a commercially available liquid-crystal coaster. They warm the material with their hands for varying lengths of time and observe the changing colors that result.

Exploding Baggie
Source Institutions
In this activity, young learners will experiment with a basic chemical reaction and observe what happens when gas gets trapped! Activity includes step-by-step instructions, safety notes and more.

Watercolor
Source Institutions
In this activity, learners will use chemistry to create a night sky watercolor painting. They will experiment to learn the effects of mixing crayon, salt, and lemon juice with water color paints.

Static Water
Source Institutions
In this activity, learners will use static elecricity to bend a stream of water without touching it. Learners will explore physics and cause and effect through this activity.

Cabbage Juice Indicator: Test the pH of household products
Source Institutions
Learners make their own acid-base indicator from red cabbage. They use this indicator to test substances around the house.

Iron in Cereal: Find iron in your food!
Source Institutions
Learners investigate an iron-fortified cereal by stirring it with a strong magnet. They discover that metallic iron is present in some cereals.

DIY Bath Bombs
Source Institutions
In this activity, learners will explore acid-base reactions and create their own bubbly results.

Snowstorm in a Jar
Source Institutions
In this activity, learners will experiment with density and chemical reactions to create a flurry activity.

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.
