Search Results
Showing results 161 to 180 of 372

Exploring Fabrication: Gummy Capsules
Source Institutions
In this activity, learners make self-assembled polymer spheres.

Kimchee Fermentation Chamber
Source Institutions
Learners make kimchee or sauerkraut, which is really just fermented cabbage, in a 2-liter plastic bottle.

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.
Gelatin Optic Fibers
Source Institutions
In this activity, learners make optical fibers out of strips of gelatin.

Frog Eggs
Source Institutions
In this activity, learners compare frog eggs to chicken eggs to better understand why frog eggs need water. Learners compare a boiled chicken egg to "frog eggs" represented by boiled tapioca.
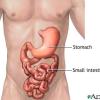
Ziploc Digestion Simulator
Source Institutions
In this biology activity, learners recreate the process of digestion in a zip lock bag. A bit of soda pop added to some crumbled crackers approximates how acids in the stomach dissolve food.

Egg-stra Strength
Source Institutions
In this physics activity, learners will investigate the strength of egg shells.

Servings and Choices
Source Institutions
In this nutrition activity (page 16 of PDF), learners document their individual eating habits and learn whether their eating patterns meet their needs.

Iodine Investigators!
Source Institutions
In this activity on page 7 of the PDF (Chemistry—It’s Elemental), learners use iodine to identify foods that contain starch.

Your Energy Needs
Source Institutions
In this activity about the relationship between food and energy (page 8 of PDF), learners estimate average daily baseline energy (Calorie) needs and energy needs for different levels of activity.
Do Plants Need Light?
Source Institutions
In this food science activity, learners conduct an experiment that demonstrates the importance of light to plants.

Dancing Spaghetti
Source Institutions
In this chemistry activity, learners use spaghetti to explore density and chemical reactions.

Make Your Own Batteries!
Source Institutions
This activity (on page 3 of the PDF under GPS: Body Electricity Activity) is a full inquiry investigation into conductivity.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.

Spit Test
Source Institutions
In this biology activity (page 8 of the PDF), learners will explore how saliva assists in the beginning of the digestive process.

Yeast-Air Balloons
Source Institutions
In this activity, learners make a yeast-air balloon to get a better idea of what yeast can do. Learners discover that the purpose of leaveners like yeast is to produce the gas that makes bread rise.

Testing Vitamin C: Chemistry's Clear Solution
Source Institutions
In this activity on page 8 of the PDF, learners investigate vitamin C. Learners conduct a chemistry experiment to determine if Tang drink mix or orange juice contains more vitamin C.

Magic Colored Milk
Source Institutions
In this chemistry activity (page 5 of the PDF), learners will use milk and a few other basic ingredients to create a chemical change to make a color wheel.

Breathing Yeasties
Source Institutions
Does yeast breathe? Find out by watching how plastic bags filled with yeast, warm water and different amounts of sugar change over time.

Try Growing Your Own Mold
Source Institutions
This is a hands-on activity that uses bread and household materials to grow mold. Learners collect dust from a room, wipe it on food, and contain it. One to seven days later, mold has grown.
