Search Results
Showing results 1 to 14 of 14

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.

Common Scents
Source Institutions
Learners use a mortar and pestle to extract clove oil from cloves using denatured alcohol. They put this oil on paper, which they can take home.

Silver Crystals
Source Institutions
This is written as a static display, but can easily become a hands-on experiment for learners.

Balloon in a Flask
Source Institutions
Learners observe a flask with a balloon attached over the mouth and inverted inside the flask.

Starch Breakdown
Source Institutions
Learners use Benedict’s solution and heat to test for the presence of simple sugars in glucose, sucrose, starch, and starch combined with amylase.
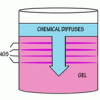
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Shake It Up!
Source Institutions
Learners observe a sealed container holding a clear colorless liquid. They shake the container and the fluid turns blue. When allowed to sit for a few moments, the fluid turns colorless again.

Lava Lamps
Source Institutions
Learners observe working lava lamps to understand how they work (included in PDF link).

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.

Phase Changes
Source Institutions
Learners observe a sealed test tube containing a small amount of solid stearic acid.

As The Stomach Churns
Source Institutions
In this chemistry activity, learners fill two test tubes with a solution of "artificial stomach fluid," consisting of hydrochloric acid in the same concentration as in human stomachs, some soap to cre

