Search Results
Showing results 1 to 20 of 24

Hot Stuff!: Investigation #4
Learners test two jars containing soil, one covered and one open, for changes in temperature. After placing the jars in the Sun, learners discover that the covered jar cools down more slowly.

Hot Stuff!: Investigation #1
Learners test two jars, one containing plain air and one containing carbon dioxide gas, to see their reactions to temperature changes.

Plugged in to CO2
Source Institutions
In this activity, learners investigate various appliances and electronics, discovering how much energy each uses and how much carbon dioxide (CO2) is released to produce that energy.

What's In Your Breath?
Source Institutions
In this activity, learners test to see if carbon dioxide is present in the air we breathe in and out by using a detector made from red cabbage.

Introduction to the Scientific Method
Source Institutions
In this activity (page 26 of the PDF), learners make observations, formulate hypotheses and design a controlled experiment, based on the reaction of carbon dioxide with calcium hydroxide.

Hot Stuff!: Testing for Carbon Dioxide from Our Own Breath
Learners blow into balloons and collect their breath--carbon dioxide gas (CO2). They then blow the CO2 from the balloon into a solution of acid-base indicator.

Breathing Blue
Source Institutions
In this activity, learners test exhaled breath for carbon dioxide and learn how to use an indicator as a simple way to measure pH.
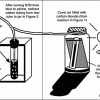
How Greenhouse Gases Absorb Heat
Source Institutions
Learners observe two model atmospheres -- one with normal atmospheric composition and another with an elevated concentration of carbon dioxide.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

Automotive Emissions and the Greenhouse Effect
Source Institutions
In this activity about global climate change, learners will conduct an experiment and collect data to compare the amount of carbon dioxide (CO2) in four different sources of gases.

Corals and Chemistry
Source Institutions
In this activity, learners investigate how increased carbon dioxide (CO2) emissions from the burning of fossil fuels is changing the acidity (pH) of the ocean and affecting coral reefs and other marin

Make a Terrarium
Source Institutions
In this activity, learners make a miniature greenhouse or "terrarium" to explore the greenhouse effect.

Fill 'er Up!
Source Institutions
Learners discover that their breath contains carbon dioxide, one of the pollutants found in car exhaust.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.

Soda Geyser
Source Institutions
In this quick activity (page 1 of PDF under SciGirls Activity: Lift Off), learners will use the ever-popular soda geyser experiment to test the reactivity of the various sugar candies or mints.

How Might Elevated CO2 Affect Plants
Source Institutions
In this activity, learners conduct an experiment to investigate the effect of elevated levels on CO2 on plant growth.

Capturing Carbon Dioxide
Source Institutions
In this activity, learners investigate carbon sequestration by creating a carbonated beverage out of apple juice and dry ice.
Coral and Chemistry
Source Institutions
In this experiment, learners will explore whether increased carbon dioxide makes our oceans more basic or more acidic.
