Search Results
Showing results 1 to 20 of 61

Cleaning with Dirt
Source Institutions
Learners build a filter from old soda bottles and dirt. They create polluted water, and pour it through their filter to clean it.

Rainbow Paper
Source Institutions
In this activity, learners will use clear nail polish and the power of chemistry to create paper with a rainbow sheen.

Say Cheese!
Source Institutions
Create a chemical reaction that makes cheese! This hands-on activity demonstrates that molecules and atoms are tiny particles that make up everything around us.

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Latent Prints
Source Institutions
In this activity, learners examine fingerprints. Learners leave a hidden print on a surface and then make their own print detecting powder from graphite (found in pencils).

Make Your Own Sculpture Dough
Source Institutions
In this activity on page 7 of the PDF, learners follow a recipe to make a dough similar to the clay artists use to make sculptures.
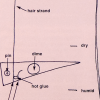
Better Hair Through Chemistry
Source Institutions
In this activity, learners hook up a hair to a lever system and create a hair hygrometer to measure changes in humidity.

Chromatography Can Separate!
Source Institutions
In this chemistry activity, learners use thin layer chromatography to determine the molecular composition of different markers.

Density: Make a golf ball float!
Source Institutions
In this activity (on page 2 of the PDF), the learner places a golf ball between salt water and colored fresh water. The golf ball is not as dense as the saltwater.

Separation Anxiety
Source Institutions
In this activity, learners discover the primary physical properties used to separate pure substances from mixtures.
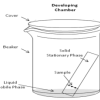
Separating with Chromatography
Source Institutions
In this experiment, learners separate different types of molecules in marker inks (using a technique called "thin layer chromatography").
Atoms and Matter (3-6)
Source Institutions
In this activity, learners build models of atoms and molecules, then consider their role in different phases of matter, density, and mixtures and solutions.

Gas Production: Blow up a balloon!
Source Institutions
In this classic reaction, learners baking soda and vinegar in a soda bottle to produce carbon dioxide (CO2) gas. This gas inflates a balloon.

Kool Colors
Source Institutions
Learners investigate how temperature affects the rate of chemical reactions by observing how steel wool reacts with various types of Kool-Aid solutions at different temperatures.

Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.

Density Rainbow
Source Institutions
In this activity, learners mix several sugar solutions to investigate the property of density. Each sugar solution has a different density and color of the rainbow.

Layered Liquids: Chemistry You Can Drink
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners make a layered drink with liquids of different densities.

Mystery Writing: Write and develop a secret message
Source Institutions
Learners write an invisible message using lemon juice on a piece of paper. They then develop the message by soaking the paper in a dilute iodine solution.

Polishing Pennies
Source Institutions
In this experiment, learners try different liquids to see which ones clean pennies best. Liquids to try include water, lemon juice, cola, vinegar, and dishwashing detergent.
