Search Results
Showing results 1 to 20 of 32

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Drying It Out
Source Institutions
In this activity, learners investigate and compare the rate of drying in different conditions.

Water Body Salinities I
Source Institutions
In this activity, learners investigate the different salinity levels of oceans, rivers and estuaries.

Sublime Sublimation
Source Institutions
In this activity, learners explore sublimation by conducting experiments with dry ice.

Moisture Makers
Source Institutions
In this outdoor activity, learners compare the moisture released from different kinds of leaves and from different parts of the same leaf, by observing the color change of cobalt chloride paper.

Sunny Day Painting
Source Institutions
In this activity, learners explore properties of water and watch evaporation happen by "painting" with water in the sun.

Separating a Mixture
Source Institutions
This activity was designed for blind learners, but all types of learners can explore means of physically separating a mixture using dissolving, filtration, and evaporation.

LEGO® Chemical Reactions
Source Institutions
This activity uses LEGO® bricks to represent atoms bonding into molecules and crystals. The lesson plan is for a 2.5 hour workshop (or four 45-minute classes).
How Does Water Climb a Tree?
Source Institutions
In this activity, learners conduct an experiment to explore how water flows up from a tree's roots to its leafy crown.

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.
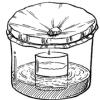
Rain Machine (Solar Still)
Source Institutions
In this activity, learners work in groups to build simple solar stills filled with salt water. After the stills are complete, learners observe what happens when they place the stills in the sun.

Leaf it to Me
Source Institutions
In this activity, learners observe the effect of transpiration as water is moved from the ground to the atmosphere.

Determining the Amount of Transpiration from a Schoolyard Tree
Source Institutions
In this activity, learners calculate the number of milliliters of water a nearby tree transpires per day.
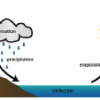
The Water Cycle
Source Institutions
Did you know that the water we use today is the same water found on Earth millions of years ago? The Earth constantly uses and recycles water in a process called the water cycle.

Liquid Crystal Thermometers
Source Institutions
In this activity, learners explore liquid crystal thermometers to observe how heat flows by conduction, convection, radiation, and evaporation.

Crystal Stencil Stars
Source Institutions
In this activity on page 6 of the PDF, learners dissolve Epsom salt in water and discover that the resulting solution can be used to create a work of art.

Cool It!
Source Institutions
In this fun hands-on activity, learners use simple materials to investigate evaporation. How can the evaporation of water on a hot day be used to cool an object? Find out the experimental way!

Veggies with Vigor
Source Institutions
In this activity, learners try to revive wilted celery. Learners discover that plants wilt when their cells lose water through evaporation. Use this activity to introduce capillary action.

What-a-cycle
Source Institutions
In this activity, learners act as water molecules and travel through parts of the water cycle to discover that it is more complex than just water moving from the ground to the atmosphere.
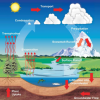
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.
