Search Results
Showing results 1 to 17 of 17

Fog Chamber
Source Institutions
In this weather-related activity, learners make a portable cloud in a bottle.

Meltdown
Source Institutions
In this activity, learners heat ice and water of the same temperature to get a hands-on look at phase changes. This is an easy and inexpensive way to introduce states of matter and thermodynamics.

Balloon Inside a Bottle
Source Institutions
In this activity about phase change and condensation, learners boil water in an empty pop bottle in the microwave.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.
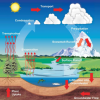
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

Use Clues to Solve an Ice Mystery
Source Institutions
Learners explore the variables that affect the properties of ice and the places where different types of ice are found.
Physical Change
Source Institutions
In this activity, learners use heat to separate zinc and copper in a penny. This experiment demonstrates physical properties and how physical change (phase change) can be used to separate matter.

Sinking Water
Source Institutions
In this experiment, learners float colored ice cubes in hot and cold water.

Keep-a-Cube
Source Institutions
In this activity, challenge learners to keep an ice cube from completely melting in 30 minutes. Learners engineer a box or wrap to prevent an ice cube from melting.

Frozen Fruit
Source Institutions
In this "Sid the Science Kid" activity from Episode 108: My Ice Pops, learners observe reversible change while thinking about ways to make ice melt.

Imploding Pop Can
Source Institutions
In this dramatic activity/demonstration about phase change and condensation, learners place an aluminum can filled with about two tablespoons of water on a stove burner.

Exploring Earth: Investigating Clouds
Source Institutions
“Exploring Earth: Investigating Clouds” is a hands-on activity in which visitors create a cloud in a bottle and explore it with laser light.

Make a Water Cycle Wristband
Source Institutions
In this activity, learners thread colored beads onto string. Each beach represent a process of the water cycle.

Can You Make Ice Cream in Two Minutes?
Source Institutions
In this demonstration, learners observe how liquid nitrogen both boils and freezes ingredients to make ice cream in two minutes.

Supercooled Water Drops
Source Institutions
In this activity, learners touch supercooled water drops with an ice crystal and trigger the water drops to freeze instantly.

Sugar/Salt Crystals
Source Institutions
In this chemistry activity (page 1 of the PDF), learners will observe a physical change.

Hot Stuff!
Source Institutions
In this activity, learners discover that sand is the major ingredient in glass.
