Search Results
Showing results 1 to 19 of 19

Hot Stuff!: Investigation #4
Learners test two jars containing soil, one covered and one open, for changes in temperature. After placing the jars in the Sun, learners discover that the covered jar cools down more slowly.

Hot Stuff!: Investigation #1
Learners test two jars, one containing plain air and one containing carbon dioxide gas, to see their reactions to temperature changes.

All Tangled Up
Source Institutions
In this activity on page 60, learners examine and simulate wildlife entanglement by experiencing what it might be like to be a marine animal trapped in debris.

Amphibian Skin
Source Institutions
In this activity, learners explore the concept of permeability to better understand why amphibians are extremely sensitive to pollution.
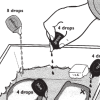
Spill Spread
Source Institutions
In this simulation, learners explore how ocean currents spread all kinds of pollution—including oil spills, sewage, pesticides and factory waste—far beyond where the pollution originates.

A Merry-Go-Round for Dirty Air
Learners build a model of a pollution control device--a cyclone. A cyclone works by whirling the polluted air in a circle and accumulating particles on the edges of the container.
Hazardous Chemicals in Your Neighborhood
Source Institutions
In this environmental science lesson, learners will examine hazardous chemicals and their effects on human health and the environment.

Hot Stuff!: Carbon Dioxide Extinguishes a Flame
In this demonstration, learners observe vinegar and baking soda creating carbon dioxide (CO2) in a bottle. The gas is poured out of a bottle onto a candle flame, putting out the candle.

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Disappearing Statues
Source Institutions
In this activity (on page 8), learners model how marble statues and buildings are affected by acid rain.

Trash Traits
Source Institutions
In this activity on page 24, learners perform experiments to examine whether or not trash can float, blow around, or wash away.

Fill 'er Up!
Source Institutions
Learners discover that their breath contains carbon dioxide, one of the pollutants found in car exhaust.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.

Water Ways
Source Institutions
In this activity (on page 2 of the PDF), learners explore surface tension by adding pennies to cups which are "full" of plain water or soapy water.

2-Liter Landfill
Source Institutions
In this activity, learners gain a better understanding of how household/school waste breaks down in a landfill. Learners collect trash and then create miniature landfills in 2-liter bottles.

Rock Bottoms
Source Institutions
Learners add acid rain (nitric acid) to two cups that represent lakes. One cup contains limestone gravel and the other contains granite gravel.

I Am/Who Has: A Litter Matching Game
Source Institutions
In this game, learners match descriptions of marine debris (shoes, batteries, paper towels, etc.) to images of these items.
Pollution in Our Watershed
Source Institutions
By building a simple watershed with paper and markers and then using a spray bottle to simulate precipitation, learners will understand how pollution accumulates in our water sources, especially from
