Search Results
Showing results 1 to 20 of 21

Collect Oxygen Over Water
Source Institutions
In this activity, learners use a pneumatic trough (see related activity) to generate and collect pure oxygen.

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.

Invisible Ink
Source Institutions
In this simple chemistry activity (page 1 of PDF under SciGirls Activity: Colorblind Dogs) about acids and bases, learners will mix a baking soda and water solution and use it to paint a message on a
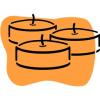
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.

Red, White and Blue I Demonstration
Source Institutions
In this chemistry demonstration, learners observe a chemical reaction that produces a colorful effect.
Rockets Away
Source Institutions
In this activity, learners build a simple "rocket" with ordinary household materials to demonstrate the basic principles behind rocketry and the principle of reaction.

Breaking Up with Combustion
Source Institutions
This activity teaches combustion as the interaction of a fuel source and oxygen.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.

Chemistry in the Kitchen
Source Institutions
In this kitchen chemistry activity, learners explore the chemistry of crystals by making sugar crystals, consider a common chemical reaction type responsible for the rising of muffins and cake in the
Gas Producing Micro-Reaction
Source Institutions
In this chemistry activity, learners use common chemicals and metals to examine reactions that produce gaseous substances.

Production of Oxygen
Source Institutions
In this chemistry activity, learners use yeast and hydrogen peroxide to generate a gas (oxygen) and test some of its properties.

Production of Hydrogen
Source Institutions
In this chemistry activity, learners use mossy zinc (or a galvanized nail) and hydrochloric acid to generate hydrogen gas and test some of its properties.
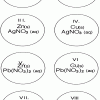
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

Double Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals to examine reactions that occur between two aqueous solutions.

Comparing the Amount of Acid in Different Solutions
Source Institutions
In this activity, learners use detergent solution to compare two solutions containing vinegar and cream of tartar.

Production of Carbon Dioxide
Source Institutions
In this chemistry activity, learners use common chemicals to produce carbon dioxide and observe its properties. This resource includes brief questions for learners to answer after the experiment.

Mixing and Unmixing in the Kitchen
Source Institutions
In this chemistry investigation, learners combine common cooking substances (flour, baking powder, sugar, salt, pepper, oil, water, food coloring) to explore mixtures.

Pop Rockets
Source Institutions
Learners place water and part of an antacid tablet in a film canister. The reaction creates a gas reaction that launches the film canister like a rocket.

Physics by the Fire: Matchstick Rocket
Source Institutions
Learners build a small rocket using a matchstick and a piece of aluminum foil. A second, lit match launches the match rocket. This activity involves fire; adult supervision required.
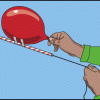
An Object in Motion
Source Institutions
In this physics activity (page nine of the pdf), learners use balloons to explore how a rocket works. It is suggested they also work to see how they can alter the velocity of the rocket.
