Search Results
Showing results 21 to 40 of 64

Fizzy Fun
Source Institutions
In this activity, learners test what happens when they put baking power on different frozen liquids.
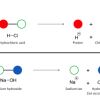
Chemical Identification
Source Institutions
In this activity, learners discover how a cabbage juice indicator helps identify acids and bases, and how iodine indicates the presence of starch.

Green Pennies
Source Institutions
In this activity, learners create their own experiment and test which of 4 mixtures of household chemicals turn pennies green over 5 days.

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

DIY Elephant Toothpaste
Source Institutions
In this activity, learners will experiment with catalysts to create an at-home version of elephant toothpaste.

Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Breaking Up with Combustion
Source Institutions
This activity teaches combustion as the interaction of a fuel source and oxygen.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.

Chemistry in the Kitchen
Source Institutions
In this kitchen chemistry activity, learners explore the chemistry of crystals by making sugar crystals, consider a common chemical reaction type responsible for the rising of muffins and cake in the

Changing Colors
Source Institutions
In this challenge, learners have to figure out in what order to combine five solutions to change the color from clear, to yellow, to blue, and back to clear.

Magic Inks
Source Institutions
Learners write their initials by applying different clear "magic ink" solutions to separate pieces of paper and then "develop" the inks with other clear solutions.

Rocket Pinwheel
Source Institutions
In this activity, learners use simple materials to construct a balloon-powered pinwheel. The pinwheel is a great way to investigate Newton's Third Law of Motion.
Gas Producing Micro-Reaction
Source Institutions
In this chemistry activity, learners use common chemicals and metals to examine reactions that produce gaseous substances.

Production of Oxygen
Source Institutions
In this chemistry activity, learners use yeast and hydrogen peroxide to generate a gas (oxygen) and test some of its properties.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.

Production of Hydrogen
Source Institutions
In this chemistry activity, learners use mossy zinc (or a galvanized nail) and hydrochloric acid to generate hydrogen gas and test some of its properties.
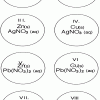
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.
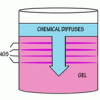
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Double Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals to examine reactions that occur between two aqueous solutions.
