Search Results
Showing results 1 to 20 of 120

Lean, Mean Information Machine: Using a Simple Model to Learn about Chromosomal DNA
Source Institutions
Learners observe a model of a cell and its chromosomal DNA made from a plastic egg and dental floss. Use this model to illustrate how much DNA is held in one cell.

Measuring Your Breathing Frequency at Rest
Source Institutions
In this activity about the brain and sleep (on page 138 of the PDF), learners measure their resting breathing rates. Learners will discover that breathing frequencies vary amongst individuals.

Look-alike Liquids
Source Institutions
Learners add drops of four liquids (water, alcohol, salt water, and detergent solution) to different surfaces and observe the liquids' behavior.

Changing the Density of a Liquid: Adding Salt
Source Institutions
Learners see that a carrot slice sinks in fresh water and floats in saltwater.

Soap: Sometimes oil and water do mix!
Source Institutions
In this activity (on page 2 of PDF), learners mix oil and water. Then, they add soap and observe what changes! The activity demonstrates how oil and water don't mix, except when soap is added.

Colors Collide or Combine
Source Institutions
Learners place multiple M&M's in a plate of water to watch what happens as the candies dissolve.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Exploring Baking Powder
Source Institutions
In this activity, learners examine baking powder, a combination of three powders: baking soda, cream of tartar, and cornstarch.

Chromatography
Source Institutions
In this activity, explore chromatography and the various colors that make up the ink in markers. Use this activity to investigate cohesion and adhesion.
Investigating Density Currents
Source Institutions
In this lab activity, learners explore how to initiate a density current. Learners measure six flasks with different concentrations of salt and water (colored blue).

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.

Divers
Source Institutions
Learners experiment with a 2-liter plastic bottle containing water and four “divers." The divers consist of open, transparent containers with the opening points downward.

Why Circulate?
Source Institutions
In this activity related to the human circulatory system (on page 10 of the PDF), learners observe the dispersion of a drop of food coloring in water, draw conclusions about the movement of dissolved

Drugs, Risks and the Nervous System
Source Institutions
In this activity, learners estimate risks associated with different events and compare their estimates to the real possibilities.

Carbon Sequestration
Source Institutions
In this inquiry-based lesson, learners measure the biomass of trees, calculate the carbon stored by the trees, and use this information to create recommendations about using trees for carbon sequestra
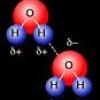
Cohesion Coin
Source Institutions
In this activity about the property of water (page 6 of the PDF), learners use a coin to demonstrate cohesion between water molecules, exploring the molecular forces that allow water molecules to "

Why Doesn’t the Ocean Freeze?
Source Institutions
In this activity, learners explore how salt water freezes in comparison to fresh water.

Release the Grease!
Source Institutions
In this simple activity (on page 7 of the PDF), learners use water and liquid dish detergent to see which one removes lipstick better from an index card.

Neutralizing Acids and Bases
Source Institutions
Learners use their knowledge of color changes with red cabbage indicator to neutralize an acidic solution with a base and then neutralize a basic solution with an acid.
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.
