Search Results
Showing results 1 to 20 of 32

Cleaning with Dirt
Source Institutions
Learners build a filter from old soda bottles and dirt. They create polluted water, and pour it through their filter to clean it.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.

Heavyweight Champion: Jupiter
Source Institutions
In this activity, learners confront their perceptions of gravity in the solar system.

Pre-School Ball Run!
Source Institutions
In this activity, learners use cardboard bases and track tubes to make a ball run to explore the properties of mass, force, and motion.

Stop the Stretching
Source Institutions
Learners work with plastic sheeting, masking tape, and string to design the perfect material for plastic chair webbing, and then construct their webbing.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

Forms of Carbon
Source Institutions
In this activity, educators can demonstrate how the nanoscale arrangement of atoms dramatically impacts a material’s macroscale behavior.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.
Properties of Metals
Source Institutions
In this activity, learners explore the properties of metals at four stations. The stations include A) Magnetism and Breakfast Cereal; B) Conductivity of Metals; C) Alloys; and D) Metal Plating.

Law of Conservation of Mass
Source Institutions
In this chemistry activity, learners explore whether matter is created or destroyed during a chemical reaction. They will compare the weight of various solutions before and after they are mixed.
Cycles in the Cards
Source Institutions
In this "game," learners explore and relate the evolution of stars to a Navajo creation story. The story is written on a series of cards, which are laid on a table as the story is told.
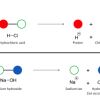
Chemical Identification
Source Institutions
In this activity, learners discover how a cabbage juice indicator helps identify acids and bases, and how iodine indicates the presence of starch.
Shrinkers
Source Institutions
Visitors use heat to shrink samples of polystyrene. They compare samples from containers that were shaped in different ways during manufacturing.

Dye Detective
Source Institutions
Learners analyze mixtures of dyes using filter paper chromatography. They place spots of the different dyes at the bottom of a piece of filter paper, and hang the paper to touch the surface of water.
Measurement: It Takes Ten
Source Institutions
In this math lesson, learners practice estimation and measurement skills as they move from station to station calculating length, volume, weight, and area.
Make Your Own DNA
Source Institutions
Learners match puzzle pieces to outlines of a DNA strand. The puzzle pieces represent the four chemicals making up DNA base pairs: adenine, thymine, guanine, and cytosine.

Flubber
Source Institutions
Learners experiment with a piece of Silly Putty® by stretching, bouncing, and snapping it. They then create flubber, a similar substance, by mixing diluted glue and a solution of sodium borate.

Off Base
Source Institutions
In this activity, learners explore the factors that tend to resist changes in pH of the ocean and why the ocean is becoming more acidic.

Recycling Rules: Understanding Recycling and a MRF
Source Institutions
In this activity, learners simulate the separation techniques that materials recovery facilities (MRFs) use and then design their own series of recycling techniques.
