Search Results
Showing results 1 to 20 of 22

Recrystallization Test
Source Institutions
In this activity, learners recrystallize substances from solutions and make observations about the resulting crystals. This test can help further identify the unknown.

Look-alike Liquids
Source Institutions
Learners add drops of four liquids (water, alcohol, salt water, and detergent solution) to different surfaces and observe the liquids' behavior.

Exploring Baking Powder
Source Institutions
In this activity, learners examine baking powder, a combination of three powders: baking soda, cream of tartar, and cornstarch.

Comparing the Density of an Object to the Density of Water
Source Institutions
Learners compare the weight of equal volumes of wax, water, and clay. Learners discover that since the wax weighs less than an equal volume of water, it is less dense than water and will float.

Twist and Spout
Source Institutions
In this activity, learners make their own "tornado" using two soda bottles and water.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Mysterious M&M's
Source Institutions
Learners place an M&M candy in water and observe what happens. The sugar-and-color coating dissolves and spreads out in a circular pattern around the M&M.

Liquid Lens
Source Institutions
In this activity, learners discover that they can create a lens from a water drop. Learners test their lens by looking at words or pictures.

Racing M&M Colors
Source Institutions
Learners design their own experiment to determine which M&M color dissolves the fastest in water.

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

Sweet Measurements
Source Institutions
In this activity on page 3 of the PDF, learners investigate how much sugar is in a soda. Learners use sugar cubes to measure and calculate the amount of sugar in a bottle of soda.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.

Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Developing Tests to Distinguish Between Similar-Looking Unknowns
Source Institutions
Learners identify an unknown liquid by comparing its behavior to known liquids. Learners drop liquids onto different surfaces and see how the liquids behave.

Safe in the Sun
Source Institutions
In this activity on page 13 of the PDF, use a special plastic card that has been painted with a chemical that changes color when it is in UV light.
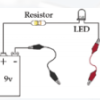
Exploring Materials: Graphene
Source Institutions
In this activity on page 4 of the PDF, explore the unique molecular structure and conductive nature of graphene. Learners construct a circuit with a battery and LED bulb.

Comparing the Amount of Acid in Different Solutions
Source Institutions
In this activity, learners use detergent solution to compare two solutions containing vinegar and cream of tartar.

Temperature Affects Dissolving
Source Institutions
Learners design their own experiment to compare how well cocoa mix dissolves in cold and hot water. They will see that cocoa mix dissolves much better in hot water. Adult supervision recommended.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

Production of a Gas: Controlling a Chemical Reaction
Source Institutions
Learners mix vinegar and baking soda to produce a gas. With the addition of a bit of liquid soap, the gas becomes trapped in measurable bubbles.
