Search Results
Showing results 641 to 660 of 2481

Color Me Blue
Source Institutions
In this activity, learners add dilute bleach solution to water that has been dyed with yellow, blue, and green food color.

Nature of Density
Source Institutions
In this activity learners will explore the concepts of density and matter in their quest to answer one simple question-will it sink or will it float?

How Boulders Are Born
Source Institutions
In this activity, learners review and discuss weathering, erosion and mass wasting, to gain a stronger understanding of how Hickory Run’s Boulder Field was formed after the Laurentide Continental Glac

Rollin’ Rollin' Rollin'
Source Institutions
In this physics activity (page 12 of the PDF), learners explore potential and kinetic energy by rolling different sized marbles down an inclined plane.
Daffy Density
Source Institutions
In this chemistry activity, learners explore density by using four solids and 6 liquids to create colorful, layered rows.

Nano Scavenger Hunt
Source Institutions
This is an activity (located on page 3 of PDF under Where's Nano? Activity) about identifying nanoscale objects and phenomena in today's world.
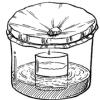
Rain Machine (Solar Still)
Source Institutions
In this activity, learners work in groups to build simple solar stills filled with salt water. After the stills are complete, learners observe what happens when they place the stills in the sun.

Finding Red
Source Institutions
In this chemistry challenge, learners systematically investigate which combination of four solutions produces a deep red color.

Skateboard Disaster
Learners examine collisions between two skateboards carrying different masses. They learn about conservation of momentum in collisions.

Design a Safer Bicycle Helmet
Source Institutions
In this activity, learners design a bicycle helmet. Participants will explore the design of bicycle helmets to gain an appreciation for the role that helmet layers play protecting the head.

Penny Drop
Source Institutions
In this quick activity about the properties of water (page 1 of PDF under SciGirls Activity: Malformed Frogs), learners will use an eyedropper to slowly place one drop of water at a time onto a penny,

Mystery Sand
Source Institutions
In this activity, learners play with surprising sand that doesn’t get wet! Learners explore how water behaves differently when it comes in contact with "magic sand" and regular sand.

Dinosaur Breath
Through discussion and hands-on experimentation, learners examine the geological (ancient) carbon cycle.

Kool Colors
Source Institutions
Learn about dyes and mordants (fixatives) when you tie-dye fabric with Kool-Aid™ and vinegar. The colored molecules in Kool-Aid™ form a chemical bond between the fiber and dye molecules.

Moving Without Wheels
In a class demonstration, learners observe a simple water cycle model to better understand its role in pollutant transport.

Silver Crystals
Source Institutions
This is written as a static display, but can easily become a hands-on experiment for learners.

Working with Watermills
Source Institutions
In this activity, learners explore how watermills have helped harness energy from water through the ages.

Balancing Sculptures
Source Institutions
In this activity, learners will use a variety of household and/or natural objects to design a sculpture that balances from a single point.

Catch a Wave: How Waves are Formed
Source Institutions
In this three-part activity, learners explore how waves are formed and why some waves are bigger than others. First, learners observe waves of water in a pan generated by an electric fan.
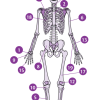
Inner Strength
Source Institutions
In this activity about endoskeletons (page 8 of PDF), learners observe, compare and contrast different kinds of chicken bones, and relate their chicken bone observations to human bones.
