Search Results
Showing results 1 to 20 of 37

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.

Luminol Test
Source Institutions
Learners mix a solution containing luminol and copper with a fake blood solution. A chemical reaction between the luminol solution and fake blood (hydrogen peroxide) show learners a blue glow.

Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.

Common Scents
Source Institutions
Learners use a mortar and pestle to extract clove oil from cloves using denatured alcohol. They put this oil on paper, which they can take home.

Build a Battery
Source Institutions
Learners build a simple one-cell battery and use an ammeter to measure the flow of current.

Silver Crystals
Source Institutions
This is written as a static display, but can easily become a hands-on experiment for learners.

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.

What's Your Blood Type?
Source Institutions
In this activity, learners perform a simulated blood test procedure.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Rocket Science
Source Institutions
Learners create a small explosion by collecting hydrogen and oxygen gas together and squeezing them into a flame.

Balloon in a Flask
Source Institutions
Learners observe a flask with a balloon attached over the mouth and inverted inside the flask.

Starch Breakdown
Source Institutions
Learners use Benedict’s solution and heat to test for the presence of simple sugars in glucose, sucrose, starch, and starch combined with amylase.
Good Vibrations
Source Institutions
In this activity, learners experiment with their voices and noisemakers to understand the connections between vibrations and the sounds created by those vibrations.

Magic Inks
Source Institutions
Learners write their initials by applying different clear "magic ink" solutions to separate pieces of paper and then "develop" the inks with other clear solutions.

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.
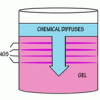
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Electroplating
Source Institutions
In this activity, learners electrically plate zinc onto brass objects.

Acid Rain
Source Institutions
In this chemistry demonstration, acid rain is simulated in a petri dish.

