Search Results
Showing results 521 to 540 of 978

Bubble Bomb
Source Institutions
Learn about chemical reactions by making a Bubble Bomb, a plastic bag you can pop with the power of fizz.

Animal Reflection Response
Source Institutions
In this activity (page 1 of the PDF under SciGirls Activity: Horse Ears), learners observe how an animal responds to its own reflection.

Sweat Spot
Source Institutions
In this activity, learners use a chemical reaction to visualize where moisture forms on the body.
Globby Gooey Gak
Source Institutions
In this activity, learners concoct some stretchy green goo called Gak. This activity will introduce learners to polymers, chemical reactions, and how scientists invent new materials.

Dispersing Dispersion
Source Institutions
In this activity, learners investigate the movement caused by dispersion. Learners discover that dispersion is the random movement of objects.

Size, Mass, Area, and Volume
Source Institutions
In this activity (page 23 of PDF), learners conduct an experiment to determine how the size and mass of a projectile affects the area and the volume of an impact crater.

Echolocation Lab
Source Institutions
In this lab, learners experience how dolphins and other echolocating animals use their senses to locate and identify objects without using their sense of sight.

Chemical Methods of Control
Source Institutions
In this lab, learners evaluate the relative effectiveness of various chemical substances (i.e. garlic powder, bathroom cleaner, mouthwash, etc.) as antimicrobial agents.

Dissolving a Substance in Different Liquids
Source Institutions
In this activity, learners make colored sugar and add it to water, alcohol, and oil to discover some interesting differences in dissolving.

Candy Chemistry
Source Institutions
In this experiment, learners test multiple food items to see if they are an acid or base using an indicator solution created with red cabbage.

Special Effects Using Household Chemicals
Source Institutions
In this activity on page 4 of the PDF (Behind the Scenes with Chemistry), learners make some special effects, including snow and breaking glass, with supplies found in the home.

Making Sodium Acetate: Hot Ice
Source Institutions
In this chemistry activity which should only be done under adult supervision (page 10 of the PDF), learners will create an exothermic process by making Sodium Acetate.

Super Soaking Materials
Source Institutions
In this activity, learners will test cups full of potting soil, sand, and sphagnum moss to see which earth material is able to soak up the most water.
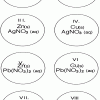
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.

Challenge: Microgravity
Source Institutions
In this activity about the circulatory system and space travel (on page 38 of the PDF), learners use water balloons to simulate the effects of gravity and microgravity on fluid distribution in the bod

Below the Surface: Surface Tension II
Source Institutions
In this activity learners explore surface tension. Why are certain objects able to float on the surface of water and how do detergents break the surface tension of water?
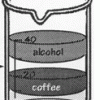
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Off Base
Source Institutions
In this activity, learners explore the factors that tend to resist changes in pH of the ocean and why the ocean is becoming more acidic.

I Can't Take the Pressure!
Learners develop an understanding of air pressure in two different activities.
