Search Results
Showing results 181 to 200 of 978

Molecules in Motion
Source Institutions
In this activity, learners add food coloring to hot and cold water to see whether heating or cooling affects the speed of water molecules.
What's So Special about Water: Absorption
Source Institutions
In this activity about water's cohesive and adhesive properties and why water molecules are attracted to each other, learners test if objects repel or absorb water.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.

Exploring How Robots Move
Source Institutions
In this activity, learners explore how pneumatics and hydraulics could be used to produce movement in a robotic arm.

Black Magic (Color Chromatography)
Source Institutions
With a coffee filter, a black marker, and a cup of water, discover the secret colors hidden in black ink.

Common Scents
Source Institutions
Learners use a mortar and pestle to extract clove oil from cloves using denatured alcohol. They put this oil on paper, which they can take home.

Testing Falling Peanut Butter Sandwich Myth
Source Institutions
In this activity related to rotational inertia (page 1 of the PDF under SciGirls Activity: Microgravity), learners will use a bit of scientific experimenting to test if open-faced peanut butter sandwi
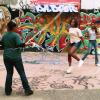
Double Dutch Distractions
Source Institutions
This activity (page 2 of the PDF under SciGirls Activity: Double Dutch) is a full inquiry investigation into whether hearing or seeing has a bigger effect on jump rope performance.
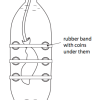
Submersibles and Marshmallows
Source Institutions
In this activity, learners discover the difficulty of ocean exploration by human beings as they investigate water pressure.

Neutralizing Acids and Bases
Source Institutions
Learners use their knowledge of color changes with red cabbage indicator to neutralize an acidic solution with a base and then neutralize a basic solution with an acid.

Copper Cleanup
Source Institutions
In this hands-on experiment, kids use chemistry to explore whether acids or bases are better at restoring a penny’s shine.
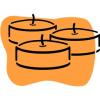
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.
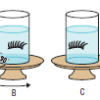
Visualizing How the Vestibular System Works
Source Institutions
In this activity (page 59 of the PDF), learners spin and observe false eyelashes in jars of water (prepared at least 1 day ahead of time) to investigate the effects of different types of motion on the

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.
Buoyancy Bulls-Eye
Source Institutions
In this hands-on activity, learners will construct a scuba diver that can float in order to explore how sea creatures stay neutrally buoyant in the ocean and to see what kinds of forces might be influ

Bubble Trouble
Source Institutions
In this activity on page 15 of the PDF, learners measure the amount of bubbles that they make using a detergent.

Stiff Bones, Bendy Bones
Source Institutions
Bones are stiff, which helps us lift heavy things and walk around, but they are also somewhat flexible, which lets them bend slightly.

LEGO® Chemical Reactions
Source Institutions
This activity uses LEGO® bricks to represent atoms bonding into molecules and crystals. The lesson plan is for a 2.5 hour workshop (or four 45-minute classes).

Mysterious M&M's
Source Institutions
Learners place an M&M candy in water and observe what happens. The sugar-and-color coating dissolves and spreads out in a circular pattern around the M&M.
