Search Results
Showing results 581 to 600 of 1185
Effects of Solar Radiation on Land and Sea
Source Institutions
In this activity, learners explore the different heating properties of soil and water.

Stuck on You: Adhesion
Source Institutions
Learners explore water adhesion and learn about why water molecules are more strongly attracted to some substances than others.

Investigating Sleep
Source Institutions
In this activity about sleep rhythms (on page 25 of the PDF), learners will investigate how changing the time they go to bed impacts their own sleep patterns.

Giving Water the Treatment
Source Institutions
In this ecology activity (page 8 of the PDF), learners explore how to filter contaminated water using a variety of materials.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.
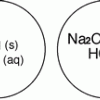
Gas Producing Micro-Reaction
Source Institutions
In this chemistry activity, learners use common chemicals and metals to examine reactions that produce gaseous substances.

Balancing Act
Source Institutions
In this physics activity (page 6 of the PDF), learners will build a class 1 lever and hypothesize and test the distances two objects need to be placed from the fulcrum in order to balance.

"Can" You Stand the Pressure
Source Institutions
In this activity about states of matter, learners get to witness firsthand the awesome power of air pressure. They watch as an ordinary soda can is crushed by invisible forces.

Marble Challenge
Source Institutions
In this physics activity (page 6 of the PDF), learners will try to keep a marble in a bottomless cup without touching the marble inside.

Iodine Investigators!
Source Institutions
In this activity on page 7 of the PDF (Chemistry—It’s Elemental), learners use iodine to identify foods that contain starch.

How does the Atmosphere keep the Earth Warmer?
Source Institutions
In this activity, learners simulate the energy transfer between the earth and space by using the light from a desk lamp desk lamp with an incandescent bulb and a stack of glass plates.

Exploring A Hydrogel
Source Institutions
In this activity on page 10 of the PDF, learners develop an experiment to answer the following question: "How much water can the hydrogel in a baby diaper hold?" Use this activity to explore polymers,

The Wave
Source Institutions
In this multi-step experiment (page 4 of pdf), learners study tide pools, and then drop a "wave" (a 4-pound bag of beans or birdseed) on a shell to mimic the force of crashing surf on tide pool animal

Water Cycle in a Bag
Source Institutions
In this activity, learners create a biosphere in a baggie.

What Causes Wind?
Source Institutions
In this sunny day experiment, learners measure and compare how quickly light and dark colored materials absorb heat.

DNA Extraction from Cheek Cells
Source Institutions
DNA is the thread of life. Encoded in its genetic sequence is the information that makes each of us unique.

Living Clocks
Source Institutions
In this activity about daily rhythms (on page 17 of the PDF), learners will explore circadian patterns in humans, animals and plants.

Try Your Hand at Nano
Source Institutions
This lesson focuses on two simple activities that younger learners can do to gain an appreciation of nanotechnology. First, learners measure their hands in nanometers.
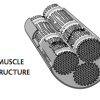
Muscle Fibers
Source Institutions
In this activity about human anatomy (page 20 of PDF), learners investigate the structure of muscles by comparing yarn and cooked meat.
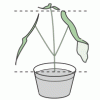
Do Plants Need Light?
Source Institutions
In this food science activity, learners conduct an experiment that demonstrates the importance of light to plants.
