Search Results
Showing results 1 to 20 of 314

Fizzy Nano Challenge
Source Institutions
This lesson focuses on how materials behave differently as their surface area increases.

Say Cheese!
Source Institutions
Create a chemical reaction that makes cheese! This hands-on activity demonstrates that molecules and atoms are tiny particles that make up everything around us.

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea
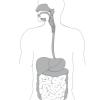
Digestion
Source Institutions
In this food science activity, learners explore digestion and proteins by observing the action of meat tenderizer on luncheon meat.

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.

Surface Area
Source Institutions
In this demonstration, learners discover that nanoparticles behave differently, in part because they have a high surface area to volume ratio.

Make Your Own Sculpture Dough
Source Institutions
In this activity on page 7 of the PDF, learners follow a recipe to make a dough similar to the clay artists use to make sculptures.
Bee Talk
Source Institutions
In this activity, learners smell bottles containing bee pheromone molecules (or herb/spice extracts as a substitute). Bees release these molecules to send messages to each other.

Collect Oxygen Over Water
Source Institutions
In this activity, learners use a pneumatic trough (see related activity) to generate and collect pure oxygen.
Trading Places: Redox Reactions
Source Institutions
Visitors add drops of copper sulfate solution onto a steel nail. They observe the nail change color from silver to brown as the copper plates onto the nail.

Find the Fizz: Discover the Secret of Baking Powder
Source Institutions
In this activity on page 4 of the PDF (Get Cooking With Chemistry), learners investigate ingredients that combine to produce gas bubbles.

Exploring Baking Powder
Source Institutions
In this activity, learners examine baking powder, a combination of three powders: baking soda, cream of tartar, and cornstarch.

Mystery Powders
Source Institutions
Learners are given mysterious white powders and have to determine their identity with chemical tests.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.

Electroplating
Source Institutions
In this electrochemistry activity, learners will explore two examples of electroplating.

Formation of a Precipitate
Source Institutions
Learners create hard water by mixing Epsom salt and water. Then they compare what happens when soap solution is mixed with hard water and regular water.

Bready Bubble Balloon
Source Institutions
Learners discover the bubble power of living cells in this multi-hour experiment with baker's yeast. Learners make a living yeast/water solution in a bottle, and add table sugar to feed the yeast.

Luminol Test
Source Institutions
Learners mix a solution containing luminol and copper with a fake blood solution. A chemical reaction between the luminol solution and fake blood (hydrogen peroxide) show learners a blue glow.
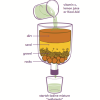
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.
