Search Results
Showing results 1 to 18 of 18

Turning the Air Upside Down: Warm Air is Less Dense than Cool Air
Learners cover a bottle with a balloon. When they immerse the bottle in warm water, the balloon inflates. When they immerse the bottle in a bowl of ice, the balloon deflates.

A Merry-Go-Round for Dirty Air
Learners build a model of a pollution control device--a cyclone. A cyclone works by whirling the polluted air in a circle and accumulating particles on the edges of the container.

How can Clouds Help Keep the Air Warmer?
Source Institutions
In this activity, learners explore how air warms when it condenses water vapor or makes clouds.
Turning the Air Upside Down: Convection Current Model
Learners see convection currents in action in this highly visual demonstration. Sealed bags of colored hot or cold water are immersed in tanks of water.

Dripping Wet or Dry as a Bone?
Learners investigate the concept of humidity by using a dry and wet sponge as a model. They determine a model for 100% humidity, a sponge saturated with water.

Hot Stuff!: Investigation #1
Learners test two jars, one containing plain air and one containing carbon dioxide gas, to see their reactions to temperature changes.
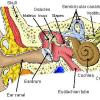
Model Eardrum
Source Institutions
In this activity (last activity on the page), learners make a model of the eardrum (also called the "tympanic membrane") and see how sound travels through the air.
Test Your Lung Power
Source Institutions
In this activity, learners try to blow up a balloon hanging inside of an empty bottle.

Lung Capacity
Source Institutions
This is an activity about lung capacity. Learners will measure their own lung capacity using a homemade spirometer.

Hot Stuff!: Carbon Dioxide Extinguishes a Flame
In this demonstration, learners observe vinegar and baking soda creating carbon dioxide (CO2) in a bottle. The gas is poured out of a bottle onto a candle flame, putting out the candle.

Hot Stuff!: Investigation #4
Learners test two jars containing soil, one covered and one open, for changes in temperature. After placing the jars in the Sun, learners discover that the covered jar cools down more slowly.

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.

Do Sweat It!
Source Institutions
In this activity, learners explore why humans sweat. Learners compare the effects of heat on a balloon filled with air and a balloon filled water.

Cartesian Diver
Source Institutions
In this demonstration, learners observe the effects of density and pressure. A "diver" constructed out of a piece of straw and Blu-Tack will bob inside a bottle filled with water.

Fungus Among Us
Source Institutions
In this environmental health activity, learners grow and observe bread mold and other kinds of common fungi over the course of 3-7 days.

Magic Wand
Source Institutions
In this activity about light and perception, learners create pictures in thin air.
