Search Results
Showing results 1 to 20 of 38
Pour Some: Measure Serving Size
Source Institutions
Make snack time into measuring time and learn to read Nutrition Facts labels. Try this when you’re using “pourable” foods, such as cereal, yoghurt, or juice.

Fireworks in a Glass
Source Institutions
In this activity, learners use water, oil, and food coloring to observe a chemical reaction that creates a shower of colors inside of a glass.

Swirling Milk
Source Institutions
In this chemistry activity, learners prepare two petri dishes, one filled with water and one filled with milk.

Underwater Fireworks
Source Institutions
In this activity, learners investigate diffusion by creating underwater "fireworks" using food coloring, oil and water.

Iron in Cereal: Find iron in your food!
Source Institutions
Learners investigate an iron-fortified cereal by stirring it with a strong magnet. They discover that metallic iron is present in some cereals.
Chemical Reactions in Your Mouth
Source Institutions
In this chemistry activity (page 5 of the PDF), learners will see that chewing is more than just the crushing up of food; there is actually a chemical change going on at the same time.

Color Splash
Source Institutions
In this activity, learners mix water, cooking oil, and liquid food coloring to create beautiful colored designs in a cup. Use this activity to explore liquid density and solubility.

Supercooled Water Drops
Source Institutions
In this activity, learners touch supercooled water drops with an ice crystal and trigger the water drops to freeze instantly.

Density Rainbows
Source Institutions
In this activity, learners explore the concept of density by pouring 5 different liquids into a jar. Food coloring is added if needed to give each liquid a distinct color.

The Amazing Water Trick
Source Institutions
Using two baby food jars, food coloring, and an index card, you'll 'marry' the jars to see how hot water and cold water mix.

Hollandaise Sauce: Emulsion at Work
Source Institutions
In this activity, learners follow a recipe to make hollandaise sauce. Learners discover how cooks use egg yolks to blend oil and water together into a smooth mix.

Layered Liquids: Chemistry You Can Drink
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners make a layered drink with liquids of different densities.

Surface Tension
Source Institutions
In this activity exploring liquid dynamics, learners design and build a clay channel in a tray of water and then see what happens when food coloring and liquid soap are added to the mix.

Floating and Sinking Fruits and Veggies
Source Institutions
In this activity, learners will explore the density of an object in water. Learners will compare what happens to fruits and vegetables in regular and salt water.
Glowing Tonic
Source Institutions
In this sunny day activity, learners compare how a cup of water and a cup of tonic water reflect or refract light in the sun.

Static Shock!
Source Institutions
In this hands-on activity, learners explore static electricity through the use of common household products. They also explore the connection between static electricity and cold weather.

Yeast-Air Balloons
Source Institutions
In this activity, learners make a yeast-air balloon to get a better idea of what yeast can do. Learners discover that the purpose of leaveners like yeast is to produce the gas that makes bread rise.
Making Waves
Source Institutions
Investigate the interaction of liquids of different densities and experiment with wave patterns with this hands-on activity.
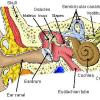
Model Eardrum
Source Institutions
In this activity (last activity on the page), learners make a model of the eardrum (also called the "tympanic membrane") and see how sound travels through the air.

Bake Ice Cream in Your Oven
Source Institutions
In this a hands-on activity, learners explore how to put ice cream in an oven without it melting. Ideas in this activity include insulation and cooking.
