Search Results
Showing results 161 to 180 of 380
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Buoyancy Bulls-Eye
Source Institutions
In this hands-on activity, learners will construct a scuba diver that can float in order to explore how sea creatures stay neutrally buoyant in the ocean and to see what kinds of forces might be influ
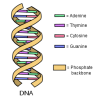
Close, Closer, Closest
Source Institutions
In this activity, learners perform an experiment that models a chromatography-like process called electrophoresis, a process used to analyze DNA.

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.

Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).
Build A Hydrometer
Source Institutions
In this activity, learners will explore how a hydrometer works by building a working model and conducting experiments.

A Pressing Engagement
Source Institutions
In this quick and easy activity and/or demonstration, learners illustrate the effect of the weight of air over our heads.

Water Fountain
Source Institutions
In this activity, learners explore how a hydraulic pump works. Learners work in teams to design and build a unique water fountain that employs a hydraulic pump.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.
What Does Life Need to Live?
Source Institutions
In this astrobiology activity (on page 11 of the PDF), learners consider what organisms need in order to live (water, nutrients, and energy).

Which Powder is It?
Source Institutions
In this chemistry challenge, learners identify an unknown white powder by comparing it with common household powders.

Plant Power
Source Institutions
In this chemistry challenge, learners identify which plants have the enzyme "catalase" that breaks hydrogen peroxide into water and oxygen.

A Dissolving Challenge
Source Institutions
In this activity, learners add objects and substances to carbonated water to discover that added objects increase the rate at which dissolved gas comes out of solution.
Investigating Convection
Source Institutions
This experiment is designed to illustrate how fluids, including water, have the ability to flow.

Lotus Leaf Effect
Source Institutions
This is a demonstration about how nature inspires nanotechnology. It is easily adapted into a hands-on activity for an individual or groups.

Single-Cell Life
Source Institutions
In this activity, learners create a soil and water model of a single-cell life environment and study living microorganisms.

Challenge: Microgravity
Source Institutions
In this activity about the circulatory system and space travel (on page 38 of the PDF), learners use water balloons to simulate the effects of gravity and microgravity on fluid distribution in the bod

Half Full or Half Empty
Source Institutions
In this activity (12th activity on the page), learners conduct an experiment to demonstrate how muscles are constantly feeding information to the brain about what they are doing.
How is Coastal Temperature Influenced by the Great Lakes and the Ocean?
Source Institutions
In this two-part lesson, learners discover how large bodies of water can serve as a heat source or sink at different times and how proximity to water moderates climate along the coast.
