Search Results
Showing results 181 to 200 of 231

Plot the Dot: A Graphical Approach to Density
Source Institutions
In this activity, learners work in groups to determine the mass and volume of four samples: glass marbles, steel washers or nuts, pieces of pine wood, and pieces of PVC pipe.

Linear Functions: Mystery Liquids
Source Institutions
In this math lesson, learners analyze the density of liquids in order to explore linear functions.

Make a Prism
Source Institutions
In this activity, learners will make their own prism and use a glass of water to separate sunlight into different colors.

Kosher Dill Current: Make Your Own Battery!
Source Institutions
This is an activity that demonstrates how batteries work using simple household materials. Learners use a pickle, aluminum foil and a pencil to create an electrical circuit that powers a buzzer.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.

Habitable Worlds
Source Institutions
In this group activity, learners consider environmental conditions—temperature, presence of water, atmosphere, sunlight, and chemical composition—on planets and moons in our solar system to determine
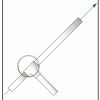
Launch Altitude Tracker
Source Institutions
In this activity, learners construct hand-held altitude trackers. The device is a sighting tube with a marked water level that permits measurement of the inclination of the tube.

Plankton Feeding
Source Institutions
This activity provides a hands-on experience with a scale model, a relatively high viscosity fluid, and feeding behaviors.

Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

Do Cities Affect the Weather?
Source Institutions
In this activity, learners explore clouds and how they form.

Critical Angle
Source Institutions
In this optics activity, learners examine how a transparent material such as glass or water can actually reflect light better than any mirror.
Good Vibrations
Source Institutions
This lesson (on pages 15-24 of PDF) explores how sound is caused by vibrating objects. It explains that we hear by feeling vibrations passing through the air.

Natural Indicators
Source Institutions
Learners combine different plant solutions -- made from fruits, vegetables, and flowers -- with equal amounts of vinegar (acid), water (neutral), and ammonia (base).

The China Hammer Mystery
Source Institutions
In this activity, learners are asked to examine the differences between two materials in a pair.

Wind Tunnel Testing
Source Institutions
In this activity, learners explore how wind tunnels provide feedback to engineers about the performance and durability of products such as planes, cars, and buildings.
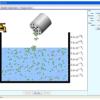
Salts & Solubility
Source Institutions
In this online interactive simulation, learners will add different salts to water and then watch the salts dissolve and achieve a dynamic equilibrium with solid precipitate.

First Impressions
Source Institutions
Learners experiment with a commercial photo-sensitive paper (Sunprint® or NaturePrint® paper). They place opaque and clear objects on the paper and expose it to bright light, observing the results.

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.

Sublimation Bubbles
Source Institutions
"Sublimation Bubbles" allows learners to explore how some solid materials, such as dry ice, can phase change directly from their solid to gaseous form.

Jell-O Model of Microfluidics
Source Institutions
This activity uses Jell-O(R) to introduce learners to microfluidics, the flow of fluids through microscopic channels.
