Search Results
Showing results 421 to 440 of 497

Push It Out
Source Institutions
In this physics related activity which requires adult supervision, learners make their own powerful water rocket and, with it, explore Newton's Third Law of Motion.
River Catcher
Source Institutions
In this activity (located at the top of the page), learners make an easy river strainer and see what they can catch.

Weather Stations: Storms
Source Institutions
In this activity, learners test how cornstarch and glitter in water move when disturbed. Learners compare their observations with videos of Jupiter's and Earth's storm movements.

Habitable Worlds
Source Institutions
In this group activity, learners consider environmental conditions—temperature, presence of water, atmosphere, sunlight, and chemical composition—on planets and moons in our solar system to determine
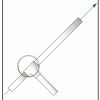
Launch Altitude Tracker
Source Institutions
In this activity, learners construct hand-held altitude trackers. The device is a sighting tube with a marked water level that permits measurement of the inclination of the tube.

Plankton Feeding
Source Institutions
This activity provides a hands-on experience with a scale model, a relatively high viscosity fluid, and feeding behaviors.
Tools of Magnification
Source Institutions
In this activity related to microbes, learners use water drops and hand lenses to begin the exploration of magnification. This activity also introduces learners to the microscope.

Comparing the Density of Different Liquids
Source Institutions
Learners carefully pour vegetable oil, water, and corn syrup in any order into a cup and discover that regardless of the order they are poured, the liquids arrange themselves in layers the same way.

Do the Mystery Samples Contain Life?
Source Institutions
In this activity (on pages 13-16 of the PDF) learners investigate three mystery samples to see which one contains life. The three samples are sand, sand and yeast, and sand and antacid.

Do Cities Affect the Weather?
Source Institutions
In this activity, learners explore clouds and how they form.

Exploring Earth: Investigating Clouds
Source Institutions
“Exploring Earth: Investigating Clouds” is a hands-on activity in which visitors create a cloud in a bottle and explore it with laser light.

Critical Angle
Source Institutions
In this optics activity, learners examine how a transparent material such as glass or water can actually reflect light better than any mirror.

Plaster Casts
Source Institutions
In this activity, learners combine two substances (plaster of Paris and water) to make a cast of an object's imprint in clay.
Good Vibrations
Source Institutions
This lesson (on pages 15-24 of PDF) explores how sound is caused by vibrating objects. It explains that we hear by feeling vibrations passing through the air.

Iridescent Art
Source Institutions
This is a quick activity (on page 2 of the PDF under Butterfly Wings Activity) that illustrates how nanoscale structures, so small they're practically invisible, can produce visible/colorful effects.

Rubber Blubber Gloves
Source Institutions
In this experiment, learners work in pairs to create two gloves -- one that contains a layer of shortening (blubber) inside, and one that doesn't.

Mystery Matter
Source Institutions
This interactive demonstration reintroduces learners to three states of matter (solid, liquid, gas), and introduces them to a fourth state of matter, plasma.

Lungometer
Source Institutions
In this environmental health activity, learners investigate their own vital lung capacities.

Hot Stuff!: Testing Ice
In this demonstration, learners compare and contrast regular water ice to dry ice (frozen carbon dioxide). Both samples are placed in a solution of acid-base indicator.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.
