Search Results
Showing results 341 to 360 of 370

Sea Level: On The Rise
Source Institutions
Learners will understand the relationship between climate change and sea-level rise.

Cabbage Juice Indicator: Test the pH of household products
Source Institutions
Learners make their own acid-base indicator from red cabbage. They use this indicator to test substances around the house.

Exploring Earth: Land Cover
Source Institutions
This activity models some of the ways natural processes, such as erosion and sediment pollution, affect Earth’s landscape.
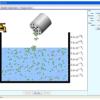
Salts & Solubility
Source Institutions
In this online interactive simulation, learners will add different salts to water and then watch the salts dissolve and achieve a dynamic equilibrium with solid precipitate.

ZOOM Glue
Source Institutions
In this activity, learners mix milk, vinegar, baking soda, and water to create sticky glue. Use this activity to explain how engineers develop and evaluate new materials and products.

Shipping for Survival
Source Institutions
In this activity, learners explore how packaging engineers develop customized shipping and packaging containers to meet the needs of many different industries.

Gelatin Used for Drug Delivery
Source Institutions
In this activity, learners discover how gelatin can be used as a medium for drug delivery. Learners create colored gelatin and then cut out pieces of the gelatin to simulate medicine (pills).

Exploring A Hydrogel
Source Institutions
In this activity on page 10 of the PDF, learners develop an experiment to answer the following question: "How much water can the hydrogel in a baby diaper hold?" Use this activity to explore polymers,

Turning the Air Upside Down: Warm Air is Less Dense than Cool Air
Learners cover a bottle with a balloon. When they immerse the bottle in warm water, the balloon inflates. When they immerse the bottle in a bowl of ice, the balloon deflates.

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.

Making Sense of Sensors
Source Institutions
In this activity, learners explore sensors and focus specifically on how to measure humidity using a sensor.

Frozen Fruit
Source Institutions
In this "Sid the Science Kid" activity from Episode 108: My Ice Pops, learners observe reversible change while thinking about ways to make ice melt.

It's a Gas, Man
Source Institutions
In this activity, learners discover if carbon dioxide has an effect on temperature.

Keep it Cool
Source Institutions
In this activity, learners explore how engineers have met the challenge of keeping foods, liquids, and other items cool.

Reusable Rockets
Source Institutions
This activity (located on page 3 of the PDF under GPS: Garbology Activity) is a full inquiry investigation into design optimization using recycled materials.

Statistics: Wet Heads
Source Institutions
In this math lesson, learners learn how to construct stem and leaf plots. Learners first estimate the number of drops of water that will fit on the head of a penny.
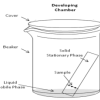
Separating with Chromatography
Source Institutions
In this experiment, learners separate different types of molecules in marker inks (using a technique called "thin layer chromatography").

Disappearing Statues
Source Institutions
In this activity (on page 8), learners model how marble statues and buildings are affected by acid rain.

Monitoring Amphibians
Source Institutions
In this field study, learners discover how to collect data in the field and how their efforts can help certain animals, specifically, amphibians.

Can You Canoe?
Source Institutions
In this activity, learners explore how engineering has impacted the manufacturing of canoes over time, including the development of new, durable, and lighter materials.
