Search Results
Showing results 121 to 140 of 301

Human Impact on Estuaries: A Terrible Spill in Grand Bay
Source Institutions
In this activity, learners make a model of a pollution spill that occurred at Bangs Lake in Mississippi and measure water quality parameters in their model.

Water Quality and pH Levels in Aquatic Ecosystems
Source Institutions
In this fun and in depth hands-on experiment, learners test various liquid samples (distilled water, lemon juice, vinegar, and baking soda mixed with water) to determine their pH levels and identify e

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p

Look-alike Liquids
Source Institutions
Learners add drops of four liquids (water, alcohol, salt water, and detergent solution) to different surfaces and observe the liquids' behavior.

M&M's in Different Temperatures
Source Institutions
Learners design their own experiment to investigate whether the temperature of the surrounding water affects the rate at which the colored coating dissolves from an M&M.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Close, Closer, Closest
Source Institutions
In this activity, learners perform an experiment that models a chromatography-like process called electrophoresis, a process used to analyze DNA.
DNA Extraction: Look at your genes!
Source Institutions
Extract your DNA from your very own cells! First, learners swish salt water in their mouth to collect cheek cells and spit the water into a glass.
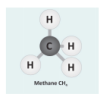
Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.

Ocean in a Bottle
Source Institutions
In this activity, learners consider how oil spills behave in the ocean and what impact they have on marine wildlife.
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).
Build A Hydrometer
Source Institutions
In this activity, learners will explore how a hydrometer works by building a working model and conducting experiments.
Foam Peanuts
Source Institutions
Learners compare the properties and solubilities of Styrofoam (TM), ecofoam packing peanuts, and popcorn. First, the solubility of each substance is tested in water.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.

Phase Changes
Source Institutions
Learners observe a sealed test tube containing a small amount of solid stearic acid.
What Does Life Need to Live?
Source Institutions
In this astrobiology activity (on page 11 of the PDF), learners consider what organisms need in order to live (water, nutrients, and energy).
