Search Results
Showing results 121 to 140 of 153

Lava Lamps
Source Institutions
Learners observe working lava lamps to understand how they work (included in PDF link).

Build a Battery
Source Institutions
Learners make a simple battery out of "sandwiches" of aluminum foil, pennies, and a salt water-soaked paper towel.

Egg Osmosis: A four day eggsperience!
Source Institutions
Eggs are placed in vinegar for one or two days to dissolve the shells. Then, learners place the eggs in water or corn syrup and observe them over a period of days.

First Impressions
Source Institutions
Learners experiment with a commercial photo-sensitive paper (Sunprint® or NaturePrint® paper). They place opaque and clear objects on the paper and expose it to bright light, observing the results.

Human Battery
Source Institutions
Learners place their hands on different metals and use an ammeter to monitor the flow of electricity from one metal to another.

Make a Totem Pole
Source Institutions
In this activity (on page 2 of PDF), learners make their own totem poles out of recycled materials.
Bend It, Break It
Source Institutions
In this activity (on pages 25-32 of PDF), learners make models of the inner ear out of pipe cleaners.
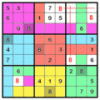
Color Sudoku
Source Institutions
The popular sudoku puzzles use numbers, but the game could played with any set of 9 different objects!

Magical Möbius
Source Institutions
In this tabletop activity (on pages 32-40), learners make Möbius strips -- 3D surfaces with only one side.

How Loud is Too Loud
Source Institutions
In this activity (described on pages 39-42 of PDF), learners make a paper wheel (on pages 57-60 of PDF) that shows them the relative loudness of different sounds.

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.

Games from Around the World
Source Institutions
This lesson (on pages 46-55 of PDF) features two strategy board games -- one from Ghana and one from China. The first game, Achi, is a cross between checkers and tic-tac-toe.
Sticky Situation
Source Institutions
In this activity, learners separate the protein from milk and and use it to make their own glue.

Paper Whites
Source Institutions
Learners observe different paper samples under ordinary room light and under a black light to learn some of the chemical differences between different types of paper.

Flubber: Make a polymer!
Source Institutions
This activity (on page 2 of the PDF) features a recipe to create the stretchy polymer Flubber from Borax detergent, white glue, and water.

Plaster Casts
Source Institutions
In this activity, learners combine two substances (plaster of Paris and water) to make a cast of an object's imprint in clay.

Shaking It!
Source Institutions
In this experiment, learners design and build a model room in a shoebox and furnish it with tiny furniture.

The Unpoppable Balloon
Source Institutions
In this activity, learners explore polymer structure and their ability to reform around objects by attempting to stab a wooden skewer through a balloon without popping it.

Brick Drop Challenge
Source Institutions
In this design challenge, leaners attempt to build a strong structure out of LEGO® bricks that can withstand a 4-foot drop.

Power To Go
Source Institutions
Learners observe an electrochemical cell constructed from a small jar containing zinc and copper strips immersed in separate solutions. The strips are connected to a motor that turns a small fan.
