Search Results
Showing results 61 to 80 of 87

Iron in Cereal: Find iron in your food!
Source Institutions
Learners investigate an iron-fortified cereal by stirring it with a strong magnet. They discover that metallic iron is present in some cereals.

What's That Sound?
Source Institutions
This game plays a dozen different sounds, altered to simulate what they would sound like if you had hearing loss.
Choose Your Ooze
Source Institutions
During this activity, learners will make different versions of "ooze" using varied proportions of detergent and glue.

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.
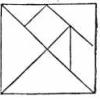
Tricky Tangrams
Source Institutions
In this activity (on pages 49-54 of PDF), learners play with tangrams, a set of triangles, squares and a parallelogram that can combine into a larger square as well as all sorts of other shapes.
Take Out the Trash
Source Institutions
Learners explore how recyclers take advantage of the different properties of materials, such as magnetism and density, to separate them from a mixture.

Dye Detective
Source Institutions
Learners use filter paper and water to analyze six different markers. They mark the paper with ink, and dip the paper in water. The water travels up the paper and dissolved ink travels with it.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.

See the Light
Source Institutions
Learners mix a solution of luminol with hydrogen peroxide to produce a reaction that gives off blue light.

Bounce vs. Thud Balls
Source Institutions
Learners compare the properties of two balls that appear identical. One ball bounces, while the other ball "thuds." The “bounce” ball is made of the polymer polybutadiene (-C4H4-).

Shake It Up!
Source Institutions
Learners observe a sealed container holding a clear colorless liquid. They shake the container and the fluid turns blue. When allowed to sit for a few moments, the fluid turns colorless again.

Dusted!
Source Institutions
Learners press their fingertip onto a clean Plexiglas sheet. The fingerprints are then revealed as learners dust over the print with fingerprint powder.

Egg Osmosis: A four day eggsperience!
Source Institutions
Eggs are placed in vinegar for one or two days to dissolve the shells. Then, learners place the eggs in water or corn syrup and observe them over a period of days.
First Impressions
Source Institutions
Learners experiment with a commercial photo-sensitive paper (Sunprint® or NaturePrint® paper). They place opaque and clear objects on the paper and expose it to bright light, observing the results.

Make a Totem Pole
Source Institutions
In this activity (on page 2 of PDF), learners make their own totem poles out of recycled materials.

Magical Möbius
Source Institutions
In this tabletop activity (on pages 32-40), learners make Möbius strips -- 3D surfaces with only one side.

Paper Whites
Source Institutions
Learners observe different paper samples under ordinary room light and under a black light to learn some of the chemical differences between different types of paper.

Flubber: Make a polymer!
Source Institutions
This activity (on page 2 of the PDF) features a recipe to create the stretchy polymer Flubber from Borax detergent, white glue, and water.

Plaster Casts
Source Institutions
In this activity, learners combine two substances (plaster of Paris and water) to make a cast of an object's imprint in clay.

Shaking It!
Source Institutions
In this experiment, learners design and build a model room in a shoebox and furnish it with tiny furniture.
