Search Results
Showing results 1 to 20 of 23

Hot Stuff!: Investigation #4
Learners test two jars containing soil, one covered and one open, for changes in temperature. After placing the jars in the Sun, learners discover that the covered jar cools down more slowly.

Hot Stuff!: Investigation #1
Learners test two jars, one containing plain air and one containing carbon dioxide gas, to see their reactions to temperature changes.
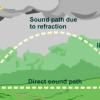
The Rumblin' Road: Determining distance to a Thunderstorm
Source Institutions
In this activity, learners discover how to determine the distance to a lightning strike or nearby thunderstorm.

How Can Gravity Make Something Go Up?
Source Institutions
In this activity, learners use cheap, thin plastic garbage bags to quickly build a solar hot air balloon. In doing so, learners will explore why hot air rises.

Wind Power: Creating a Wind Generator
Source Institutions
This lesson challenges groups of learners to design and construct a wind generator with the most electrical output.

That Sinking Feeling
Source Institutions
In this quick activity, learners observe how salinity and temperature affect the density of water, to better understand the Great Ocean Conveyor.

Exploring Moisture on the Outside of a Cold Cup
Source Institutions
In this activity, learners explore the relationship between cooling water vapor and condensation. Learners investigate condensation forming on the outside of a cold cup.

Can Crushers
Source Institutions
In this activity, learners conduct an experiment by heating an aluminum can filled with water to investigate air pressure.

Fly a Hot-Air Balloon
Source Institutions
Learners assemble a hot-air balloon from tissue paper. The heated air (from a heat gun) inside the balloon is less dense than the surrounding air and causes the balloon to float.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Balloon Inside a Bottle
Source Institutions
In this activity about phase change and condensation, learners boil water in an empty pop bottle in the microwave.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Toasty Wind
Source Institutions
In this quick activity, learners use a toaster to investigate the source for the Earth's wind. Learners hold a pinwheel above a toaster to discover that rising heat causes wind.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.
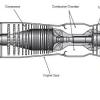
Jet Propulsion
Source Institutions
In this two-part activity, learners work in pairs to examine the four basic stages of a turbine engine.

If Hot Air Rises, Why is it Cold in the Mountains?
Source Institutions
This demonstration/activity helps learners understand why higher elevations are not always warm simply because "hot air rises." Learners use a tire pump to increase the pressure and temperature inside

Sizing Up Temperature
Source Institutions
In this activity, learners explore Charles' Law in a syringe.

Full of Hot Air: Hot Air Balloon Building
Source Institutions
In this activity, learners create a model of a hot air balloon using tissue paper and a hairdryer. Educators can use this activity to introduce learners to density and its role in why things float.

Imploding Pop Can
Source Institutions
In this dramatic activity/demonstration about phase change and condensation, learners place an aluminum can filled with about two tablespoons of water on a stove burner.

Amazing Air
Source Institutions
In this activity, learners construct a small "air cannon," and use its airflow to put out a candle (lit with the help of an adult).
