Search Results
Showing results 1 to 20 of 36

Say Cheese!
Source Institutions
Create a chemical reaction that makes cheese! This hands-on activity demonstrates that molecules and atoms are tiny particles that make up everything around us.
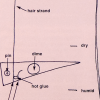
Better Hair Through Chemistry
Source Institutions
In this activity, learners hook up a hair to a lever system and create a hair hygrometer to measure changes in humidity.

Chromatography Can Separate!
Source Institutions
In this chemistry activity, learners use thin layer chromatography to determine the molecular composition of different markers.

Density: Make a golf ball float!
Source Institutions
In this activity (on page 2 of the PDF), the learner places a golf ball between salt water and colored fresh water. The golf ball is not as dense as the saltwater.

Digit's Cyber-Dough
Source Institutions
In this fun hands-on activity, learners whip up a batch of cyber-dough (play dough) using math for measurements.

Separation Anxiety
Source Institutions
In this activity, learners discover the primary physical properties used to separate pure substances from mixtures.
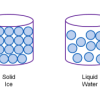
Atoms and Matter (3-6)
Source Institutions
In this activity, learners build models of atoms and molecules, then consider their role in different phases of matter, density, and mixtures and solutions.

DNA Modeling Activity
Source Institutions
Using pipe cleaners, straws, and beads, learners explore the building blocks of life by creating their own model of DNA.

Mystery Powders
Source Institutions
In this activity on page 2 of the PDF (Get Cooking With Chemistry), learners conduct chemical tests on certain powders used in cooking.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.

Layered Liquids: Chemistry You Can Drink
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners make a layered drink with liquids of different densities.

Polishing Pennies
Source Institutions
In this experiment, learners try different liquids to see which ones clean pennies best. Liquids to try include water, lemon juice, cola, vinegar, and dishwashing detergent.

Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.
Penny for Your Thoughts
Source Institutions
In this activity, learners will explore how metals react with each other. They will see these metals change before their eyes as they coat a paperclip with the copper taken from a penny.

Crystals: Grow Your Own Garden
Source Institutions
In this simple activity (on page 2 of the PDF), learners make a crystal garden using salt, water, and a brick.

Water Ways
Source Institutions
In this activity (on page 2 of the PDF), learners explore surface tension by adding pennies to cups which are "full" of plain water or soapy water.

Iron in the Environment
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners corrode a penny in a cup with vinegar, salt water, and a source of iron (nails, paper clips, or twist ties).

Acids & Bases
Source Institutions
In this activity, learners test the pH of safe liquids available at home by creating a pH indicator from mashed blueberries.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.

Invisible Ink
Source Institutions
In this hands-on activity (on page 2 of the PDF), learners experiment with lemon juice and paper to create a message that can only be revealed using chemistry.
