Search Results
Showing results 1 to 20 of 39

Matter on the Move
Source Institutions
Learners observe and conduct experiments demonstrating the different properties of hot and cold materials.

Collect Oxygen Over Water
Source Institutions
In this activity, learners use a pneumatic trough (see related activity) to generate and collect pure oxygen.

Find the Fizz: Discover the Secret of Baking Powder
Source Institutions
In this activity on page 4 of the PDF (Get Cooking With Chemistry), learners investigate ingredients that combine to produce gas bubbles.

Mystery Powders
Source Institutions
Learners are given mysterious white powders and have to determine their identity with chemical tests.

Jam Jar Jet
Source Institutions
In this activity, learners create a "Jam Jar Jet" based on Francois Reynst's discovery of a pulsejet engine, which uses one opening for both air intake and exhaust.

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.
Gas Production: Blow up a balloon!
Source Institutions
In this classic reaction, learners baking soda and vinegar in a soda bottle to produce carbon dioxide (CO2) gas. This gas inflates a balloon.
Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.

3-2-1 POP!
Source Institutions
In this physics activity, learners build their own rockets out of film canisters and construction paper.

Inflate-a-mole
Source Institutions
In this activity, learners conduct an experiment to find the volume of one mole of gas. Learners capture sublimated gas from dry ice in a ziploc bag and use water displacement to measure its volume.
Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

A Feast for Yeast
Source Institutions
In this activity on page 6 of the PDF (Get Cooking With Chemistry), learners investigate yeast. Learners prepare an experiment to observe what yeast cells like to eat.

Balloon in a Flask
Source Institutions
Learners observe a flask with a balloon attached over the mouth and inverted inside the flask.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Conservation of Mass
Source Institutions
This activity was designed for blind learners, but all types of learners can participate to learn about conservation of gas. This is one of the classic experiments using baking soda and vinegar.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.
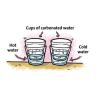
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Air, It's Really There
Source Institutions
This lesson focuses on molecular motion in gases. Learners compare the mass of a basketball when it is deflated and after it has been inflated.

Soda Geyser
Source Institutions
In this quick activity (page 1 of PDF under SciGirls Activity: Lift Off), learners will use the ever-popular soda geyser experiment to test the reactivity of the various sugar candies or mints.

Mixing and Unmixing in the Kitchen
Source Institutions
In this chemistry investigation, learners combine common cooking substances (flour, baking powder, sugar, salt, pepper, oil, water, food coloring) to explore mixtures.
