Search Results
Showing results 1 to 10 of 10

Pea Brain!: Explorations in Estimation
Source Institutions
In this activity, learners use two different techniques to estimate how many little things fit into one bigger thing.

Indicating Electrolysis
Source Institutions
In this activity, learners build a simple electrolysis device. Then learners use an indicating solution to visualize hydrogen and oxygen molecules in water.

Sticky Snot
Source Institutions
In this activity, learners create slime to model mucus and examine how it collects simulated particles. Mucus keeps particles from the environment out of our lungs when we breathe.

Smelly Balloons
Source Institutions
Are balloons porous or non-porous? In this activity, learners watch an entertaining Mr. O video and conduct a simple experiment to find out.

Limewater
Source Institutions
This is a chemistry lab activity about solutions (page 6 of the PDF). Students make a limewater testing solution for carbon dioxide and explore the concepts of solubility and precipitates.

Holding Charge
Source Institutions
In this trick, learners discover how to stick a straw to the palm of their hand, window door, or anywhere using static electricity.
Beam Me Up!
Source Institutions
This is a quick activity (on page 2 of the PDF under Stained Glass Activity) about the "Tyndall effect," the scattering of visible light when it hits very small dispersed particles.

Conductivity Meter
Source Institutions
In this activity, learners build a simple qualitative conductivity tester with a battery, bulb and foil.
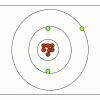
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).
Filtering
Source Institutions
Make a quick and easy filter from household materials. A filter will catch any solids suspended in a liquid and filter them out. By using a filter, learners can discover amazing things.
