Search Results
Showing results 1 to 20 of 22

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Invisible Ink
Source Institutions
In this simple chemistry activity (page 1 of PDF under SciGirls Activity: Colorblind Dogs) about acids and bases, learners will mix a baking soda and water solution and use it to paint a message on a

Rate of Solution Demonstration
Source Institutions
In this chemistry demonstration, learners investigate the factors that increase the rate of dissolution for a solid.

Indicating Electrolysis
Source Institutions
In this activity, learners build a simple electrolysis device. Then learners use an indicating solution to visualize hydrogen and oxygen molecules in water.

We all Scream for Ice Cream
Source Institutions
In this activity, learners observe how salinity affects the freezing point of water by making and enjoying ice cream.

Indicator Paper
Source Institutions
Use grape juice, baking soda, water and vinegar to make acid and base indicator paper! This activity contains a recipe and instructions for the indicator paper.

Crystal Creations: Grow Spikes of Crystals in the Sun
Source Institutions
This activity shows you how to make amazing crystal spikes using Epsom salt and the sun.
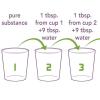
Sniffing for a Billionth
Source Institutions
This is an activity (located on page 4 of the PDF under What's Nano? Activity) about size and scale.

Chemistry in the Kitchen
Source Institutions
In this kitchen chemistry activity, learners explore the chemistry of crystals by making sugar crystals, consider a common chemical reaction type responsible for the rising of muffins and cake in the

Dissolving a Substance in Different Liquids
Source Institutions
In this activity, learners make colored sugar and add it to water, alcohol, and oil to discover some interesting differences in dissolving.

Grow Rock Candy
Source Institutions
Learners grow sugar crystals (rock candy). They make a hot solution that has an excess of sugar dissolved in it, then as the solution cools, they see sugar crystals form.

Make Your Own Soda Pop
Source Institutions
In this chemistry activity (page 8 of the PDF), learners will identify the instances of physical change, chemical change, and solutions while making homemade soda pop.

Resistance is Useful
Source Institutions
Learners write or draw with white crayon on white paper. They look and feel to detect their marks on the paper. Then, learners paint over their paper with watercolor paint.

Comparing Crystals
Source Institutions
In this chemistry activity (page 3 of the PDF), learners will learn about crystals by growing their very own.

Light Soda
Source Institutions
In this activity, learners sublimate dry ice and then taste the carbon dioxide gas.

Pages of a Forbidden Tome
Source Institutions
In this activity, learners use chemistry to produce weathered "antiqued" paper with burned edges. Learners first soak white paper in coffee and then apply a charring solution of ammonium chloride.

Limewater
Source Institutions
This is a chemistry lab activity about solutions (page 6 of the PDF). Students make a limewater testing solution for carbon dioxide and explore the concepts of solubility and precipitates.

Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.
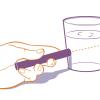
Beam Me Up!
Source Institutions
This is a quick activity (on page 2 of the PDF under Stained Glass Activity) about the "Tyndall effect," the scattering of visible light when it hits very small dispersed particles.

Conductivity Meter
Source Institutions
In this activity, learners build a simple qualitative conductivity tester with a battery, bulb and foil.
