Search Results
Showing results 81 to 100 of 119

Energetic Water
Source Institutions
In this activity, learners explore how hot and cold water move. Learners observe that temperature and density affect how liquids rise and fall.

Feel the Heat
Source Institutions
In this design challenge activity, learners design and build a solar hot water heater. Their goal is to create a heater that yields the highest temperature change.

How Many Pennies?
Source Institutions
In this activity (pages 13-14), learners investigate the properties of smart materials, which are materials that respond to things that happen around them.

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Keep-a-Cube
Source Institutions
In this activity, challenge learners to keep an ice cube from completely melting in 30 minutes. Learners engineer a box or wrap to prevent an ice cube from melting.
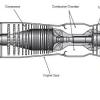
Jet Propulsion
Source Institutions
In this two-part activity, learners work in pairs to examine the four basic stages of a turbine engine.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

What is a "Model"?
Source Institutions
In this activity, learners simulate the behavior of the atmosphere.

Wintergreen
Source Institutions
In this outdoor, winter activity, learners find living green plants under the snow and determine the light and temperature conditions around the plants.

Doghouse Design
Source Institutions
This activity (on page 2 of the PDF under SciGirls Activity: Doghouse Design) is a full inquiry investigation into absorption and reflection of radiant energy.

Instant Ice Cream with a Dry Ice Bath
Source Institutions
In this chemistry meets cooking activity, learners make carbonated, vanilla ice cream using dry ice and denatured ethanol, which are both inexpensive and accessible.

What does Color have to do with Cooling?
Source Institutions
In this demonstration/experiment, learners discover that different colors and materials (metals, fabrics, paints) radiate different amounts of energy and therefore, cool at different rates.

Pie-Pan Convection
Source Institutions
It's difficult to see convection currents in any liquid that's undergoing a temperature change, but in this Exploratorium Science Snack, you can see the currents with the help of food coloring.

Sizing Up Temperature
Source Institutions
In this activity, learners explore Charles' Law in a syringe.

Frozen Fruit
Source Institutions
In this "Sid the Science Kid" activity from Episode 108: My Ice Pops, learners observe reversible change while thinking about ways to make ice melt.
Cooling the Mummy's Tomb
Source Institutions
In this activity, learners conduct an experiment to help Pharaoh design a better insulated tomb.

It's a Gas, Man
Source Institutions
In this activity, learners discover if carbon dioxide has an effect on temperature.

Get the Porridge Just Right
Source Institutions
Learners set up three different bowls, each with a different mass of oatmeal. Learners monitor the temperature of the oatmeal and find that larger masses take longer to cool.

Illuminations on Rates of Reactions
Source Institutions
In this activity, learners investigate the speed of chemical reactions with light sticks. Learners discover that reactions can be sped up or slowed down due to temperature changes.

Lava Lamps
Source Institutions
Learners observe working lava lamps to understand how they work (included in PDF link).
