Search Results
Showing results 41 to 60 of 293

Inkjet Printer
Source Institutions
In this activity, learners investigate how inkjet printers produce tiny, precise drops of ink.

Evaporation
Source Institutions
This three-part activity consists of an activity that groups of learners develop themselves, a given procedure, and an optional demonstration.

Eddy Currents
Source Institutions
In this activity related to magnetism and electricity, learners discover that a magnet falls more slowly through a metallic tube than it does through a nonmetallic tube.

Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.

Diffraction
Source Institutions
In this optics activity, demonstrate diffraction using a candle or a small bright flashlight bulb and a slide made with two pencils.
Pepper Scatter
Source Institutions
In this activity, learners explore the forces at work in water. Learners experiment to find out what happens to pepper in water when they touch it with bar soap and liquid detergent.
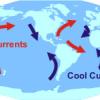
How it is Currently Done
Source Institutions
In this quick activity, learners observe how wind creates ocean currents.

Toilet Model
Source Institutions
In this activity, PVC pipe, plastic water bottles and vinyl tubing are used to make a simple working toilet model. The model shows the role of a siphon in the flushing of a toilet.

Atmospheric Collisions
Source Institutions
In this activity/demonstration, learners observe what happens when two ping pong balls are suspended in the air by a hair dryer. Use this activity to demonstrate how rain drops grow by coalescence.

Red, White and Blue II Demonstration
Source Institutions
In this chemistry demonstration, learners investigate the rule "likes dissolve likes" by combining three, immiscible liquids to create a colorful density column.

Erupting Fizz
Source Institutions
This is a highly visual demonstration that illustrates both the effects of density and chemical reactions.

Loony Balloons
Source Institutions
In this activity, learners investigate how changing the center of gravity of a balloon affects how it travels. Learners fill a balloon with a little bit of water and insert into an empty balloon.

Magical Match
Source Institutions
In this demonstration, learners will be "wowed" as three matches burn to form a triangular pyramid shape and "magically" rise off the table.

Moonlight Serenade
Source Institutions
In this activity, learners act as the Earth and observe how different angles between the Sun, Earth, and Moon affect the phases of the moon we see each month.

Springs and Stomachs
Source Institutions
In this demonstration, learners investigate mass, gravity, and acceleration by dropping a wooden bar with a balloon attached to its underside, a mass suspended from it by rubber bands, and a sharp-poi

Common Scents
Source Institutions
Learners use a mortar and pestle to extract clove oil from cloves using denatured alcohol. They put this oil on paper, which they can take home.
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.
Become a Master of Inertia
Source Institutions
In this activity, learners explore inertia as they attempt to whip a strip of paper out from under two coins dangling on the rim of a water glass.

Parabolas: It's All Done with Mirrors
Source Institutions
In this activity about light and reflection, learners use a special device called a Mirage Maker™ to create an illusion.

Red, White and Blue I Demonstration
Source Institutions
In this chemistry demonstration, learners observe a chemical reaction that produces a colorful effect.
