Search Results
Showing results 1 to 20 of 90

Tiny Tubes
Source Institutions
In this activity, learners make "totally tubular" forms of carbon. Learners use chicken wire to build macro models of carbon nanotubes.
Mercury in the Environment
Source Institutions
In this environmental science lesson, learners will examine the dangers of mercury and how humans contribute to growing mercury emissions on Earth.

Magnets on the Move
Source Institutions
In this activity, learners investigate the behavior of magnets. Learners create a "wonder wand" with a magnet so they can move a skater around.

Best Bubbles
Source Institutions
In this activity, learners experiment with creating various types of bubble solutions and testing which ingredients form longer-lasting bubbles.

Electroplating
Source Institutions
In this electrochemistry activity, learners will explore two examples of electroplating.
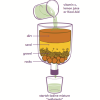
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Formulas Poker
Source Institutions
In this adapted version of poker, learners practice writing chemical formulas by playing this chemistry card game.

Sublime Sublimation
Source Institutions
In this activity, learners explore sublimation by conducting experiments with dry ice.

Plugged in to CO2
Source Institutions
In this activity, learners investigate various appliances and electronics, discovering how much energy each uses and how much carbon dioxide (CO2) is released to produce that energy.

Spectroscope
Source Institutions
In this activity (posted on March 12, 2011), learners follow the steps to construct a spectroscope, a tool used to analyze light and color.

Jiggly Jupiter
Source Institutions
In this activity, learners build edible models of Jupiter and Earth to compare their sizes and illustrate the planets' internal layers.

The Colors of Flowers
Source Institutions
In this activity, learners perform an experiment to find out what determines a flower's color.

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.

Fast Rusting
Source Institutions
In this activity, learners conduct an experiment to find out if steel wool will weigh more or less when it is burned. Learners will explore the effects of oxidation and rusting on the steel wool.

LEGO® Chemical Reactions
Source Institutions
This activity uses LEGO® bricks to represent atoms bonding into molecules and crystals. The lesson plan is for a 2.5 hour workshop (or four 45-minute classes).
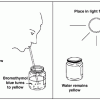
Carbon Dioxide Removal
Source Institutions
In this experiment using sprigs of Elodea, learners will observe a natural process that removes carbon dioxide (CO2) from Earth's atmosphere.

Forms of Carbon
Source Institutions
In this activity, educators can demonstrate how the nanoscale arrangement of atoms dramatically impacts a material’s macroscale behavior.

Mystery Matter
Source Institutions
This interactive demonstration reintroduces learners to three states of matter (solid, liquid, gas), and introduces them to a fourth state of matter, plasma.

Dye Like A Natural
Source Institutions
In this activity, learners stain fabrics--on purpose!

Polyatomic Ion Bingo
Source Institutions
This activity was designed for blind learners, but all types of learners can play this game to learn about the major polyatomic ions (an ion that consists of two different elements).
