Search Results
Showing results 241 to 260 of 397

A Recipe for Air
Learners use M&Ms® (or any other multi-color, equally-sized small candy or pieces) to create a pie graph that expresses the composition of air.

Jiggly Jupiter
Source Institutions
In this activity, learners build edible models of Jupiter and Earth to compare their sizes and illustrate the planets' internal layers.

Joe's Place
Source Institutions
In this math activity (Page 8 of the Dining Out! PDF), younger learners select items from a menu and count out the total amount needed using the fewest bills and coins possible.

The Thousand-Yard Model
Source Institutions
This is a classic exercise for visualizing the scale of the Solar System.

Dye Like A Natural
Source Institutions
In this activity, learners stain fabrics--on purpose!
How Does Water Climb a Tree?
Source Institutions
In this activity, learners conduct an experiment to explore how water flows up from a tree's roots to its leafy crown.

Ziptop Bag Chemistry
Source Institutions
In this chemistry activity, learners perform three chemical reactions in a sealed zip-top bag. Learners will record their observations and classify the changes as chemical or physical.

Wheat Evolution: Dough Rising and Baking
Source Institutions
In this activity (Page 25 of PDF), learners investigate the evolution of wheat by creating dough from different flours, observing the samples of dough as they rise, and then baking the dough.

How Boulders Are Born
Source Institutions
In this activity, learners review and discuss weathering, erosion and mass wasting, to gain a stronger understanding of how Hickory Run’s Boulder Field was formed after the Laurentide Continental Glac

Wheat Germ DNA Extraction
Source Institutions
This laboratory exercise is designed to show learners how DNA can easily be extracted from wheat germ using simple materials.
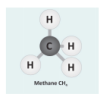
Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.
Green Travelers
Source Institutions
In this activity (on pages 23-29), partners use the Plant Traveler Cards, along with a world map and map worksheets, to follow plants such as cassava, chocolate and coffee that grew first in one part

Onion DNA Extraction
Source Institutions
This laboratory exercise is designed to show learners how DNA can easily be extracted from onion cells using simple materials.

Mixtures and Solutions
Source Institutions
This activity was designed for blind learners, but all types of learners can use it to investigate heterogeneous and homogeneous mixtures and solutions, identify the differences, and explore the conce

Dissolving Different Liquids in Water
Source Institutions
In this activity, learners add different liquids to water and apply their working definition of “dissolving” to their observations.
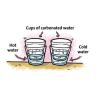
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Exploring Fabrication: Gummy Capsules
Source Institutions
In this activity, learners make self-assembled polymer spheres.

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.

Scent Tag
Source Institutions
In this matchmaking activity (on page 2 of the PDF under GPS: Animal Scent Activity), learners will each have a scented cotton ball taped to their shoulder. The scent (e.g.
