Search Results
Showing results 1 to 20 of 27

Burn a Peanut
Source Institutions
In this activity, learners burn a peanut, which produces a flame that can be used to boil away water and count the calories contained in the peanut.

Candy Chemosynthesis
Source Institutions
In this activity, groups of learners work together to create edible models of chemicals involved in autotrophic nutrition.

Plankton Feeding
Source Institutions
This activity provides a hands-on experience with a scale model, a relatively high viscosity fluid, and feeding behaviors.

Cabbage Juice Indicator
Source Institutions
In this chemistry activity, learners make indicator solution from red cabbage. Then, learners test everyday foods and household substances using the cabbage juice indicator.

Biotech in a Bag
Source Institutions
In a series of three experiments, learners explore the basics of biotechnology using self-locking plastic baggies. Each experiment demonstrates a phenomenon or principle of biotechnology.

Cake by Conduction
Source Institutions
In this demonstration, cook a cake using the heat produced when the cake batter conducts an electric current.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!

Rate of Solution Demonstration
Source Institutions
In this chemistry demonstration, learners investigate the factors that increase the rate of dissolution for a solid.
Are you a Supertaster?
Source Institutions
In this activity, learners examine their tongue and taste buds.

Diffusion of Water with Gummy Bears
Source Institutions
In this activity, learners investigate the movement of water into and out of a polymer. Learners test the diffusion of water through gummy bears, which are made of sugar and gelatin (a polymer).
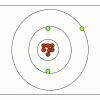
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).

Get the Porridge Just Right
Source Institutions
Learners set up three different bowls, each with a different mass of oatmeal. Learners monitor the temperature of the oatmeal and find that larger masses take longer to cool.

Adherence to HIV Treatment
Source Institutions
In this activity, learners simulate taking HIV antiretroviral drugs by using Tic Tac mints and Kool-Aid packets.

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.

Dye Like A Natural
Source Institutions
In this activity, learners stain fabrics--on purpose!

Ziptop Bag Chemistry
Source Institutions
In this chemistry activity, learners perform three chemical reactions in a sealed zip-top bag. Learners will record their observations and classify the changes as chemical or physical.

Having a Gas with Cola
Source Institutions
In this activity, learners measure the amount of carbon dioxide in a carbonated drink.

Exponential Models: Rhinos and M&M’s ®
Source Institutions
In this math lesson, learners model exponential decay and exponential growth using M&M's, paper folding, and African rhino population data.
Multi-Variable Relations: Stressed to the Breaking Point
Source Institutions
In this math lesson, learners explore the relationship between the thickness of a spaghetti bridge, the length of the bridge, and the amount of weight that can be supported by the bridge.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.
