Search Results
Showing results 61 to 80 of 168

Shimmering Lenses
Source Institutions
In this activity, learners use Jell-O to explore lenses. Learners cut Jell-O into convex and concave lens shapes and examine how light exits each lens in a darkened room.

Crunch and Munch Lab
Source Institutions
In this activity, learners use three types of cheesy snacks--cheese balls, cheese puffs, and Cheetos--to learn about polymers.

Light Soda
Source Institutions
In this activity, learners sublimate dry ice and then taste the carbon dioxide gas.

Isolation of DNA from Onion
Source Institutions
This laboratory exercise is designed to show learners how DNA can easily be extracted from onion cells. It includes an optional test for the presence of DNA.

Gelatin Prism
Source Institutions
In this activity, learners make prisms from gelatin. Learners then shine light through the prisms and discover what happens. This activity introduces learners to the idea of refraction.

How Sweet It Is
Source Institutions
In this activity (4th activity on the page), learners use their sense of smell to rate and arrange containers filled with different dilutions of a scent (like cologne or fruit juice) in order from wea

One In The Hand
Source Institutions
In this physics demonstration, learners are challenged to break a raw egg just by squeezing it. Learners will be shocked by their inability to complete the deceivingly simple challenge.

Rate of Solution Demonstration
Source Institutions
In this chemistry demonstration, learners investigate the factors that increase the rate of dissolution for a solid.

Leaning Tower of Pasta
Learners build structures from spaghetti and marshmallows to determine which structures are able to handle the greatest load.

Hot & Cold
Source Institutions
In this activity, learners experiment with hydrogen peroxide, vinegar, yeast, and baking soda to produce hot and cold reactions. Use this activity to demonstrate exothermic and endothermic reactions.

Space Jell-O
Source Institutions
Albert Einstein proved that space bends around anything that has mass. This activity uses Jell-O's ability to bend around objects as a model for space bending around planets and stars.

Diffusion of Water with Gummy Bears
Source Institutions
In this activity, learners investigate the movement of water into and out of a polymer. Learners test the diffusion of water through gummy bears, which are made of sugar and gelatin (a polymer).
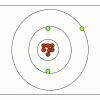
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).

Nano Ice Cream
Source Institutions
In this activity/demo, learners discover how liquid nitrogen cools a creamy mixture at such a rapid rate that it precipitates super fine grained (nano) ice cream.

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Get the Porridge Just Right
Source Institutions
Learners set up three different bowls, each with a different mass of oatmeal. Learners monitor the temperature of the oatmeal and find that larger masses take longer to cool.

Have Your DNA and Eat It Too
Source Institutions
In this activity, learners build edible models of DNA, while learning basic DNA structure and the rules of base pairing.

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.
Air-filled (Pneumatic) Bone Experiments
Source Institutions
Just like birds, some dinosaurs had air-filled (pneumatic) bones, which made the dinosaurs' skeletons lighter.

Lifting Lemon
Source Institutions
In this physics demonstration, learners will be surprised when a lemon slice appears to magically levitate within a pint glass.
